Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-02-28 , DOI: 10.1016/j.bmcl.2018.02.048 Nagarajan Muthukaman , Sanjay Deshmukh , Macchindra Tambe , Dnyandeo Pisal , Shital Tondlekar , Mahamadhanif Shaikh , Neelam Sarode , Vidya G. Kattige , Pooja Sawant , Monali Pisat , Vikas Karande , Srinivasa Honnegowda , Abhay Kulkarni , Dayanidhi Behera , Satyawan B. Jadhav , Ramchandra R. Sangana , Girish S. Gudi , Neelima Khairatkar-Joshi , Laxmikant A. Gharat
|
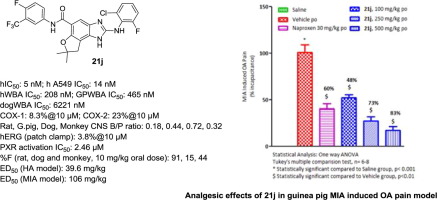
In an effort to identify CYP and hERG clean mPGES-1 inhibitors from the dihydrofuran-fused tricyclic benzo[d]imidazole series lead 7, an extensive structure-activity relationship (SAR) studies were performed. Optimization of A, D and E-rings in 7 afforded many potent compounds with human whole blood potency in the range of 160–950 nM. Selected inhibitors 21d, 21j, 21m, 21n, 21p and 22b provided selectivity against COX-enzymes and mPGES-1 isoforms (mPGES-2 and cPGES) along with sufficient selectivity against prostanoid synthases. Most of the tested analogs demonstrated required metabolic stability in liver microsomes, low hERG and CYP liability. Oral pharmacokinetics and bioavailability of lead compounds 21j, 21m and 21p are discussed in multiple species like rat, guinea pig, dog, and cynomolgus monkey. Besides, these compounds revealed low to moderate activity against human pregnane X receptor (hPXR). The selected lead 21j further demonstrated in vivo efficacy in acute hyperalgesia (ED50: 39.6 mg/kg) and MIA-induced osteoarthritic pain models (ED50: 106 mg/kg).
中文翻译:

通过优化二氢呋喃稠合的三环苯并[ d ]咪唑系列的结构来减轻CYP和hERG的责任-有效,选择性和口服有效的微粒体前列腺素E合酶-1(mPGES-1)抑制剂:第2部分
为了从二氢呋喃稠合的三环苯并[ d ]咪唑系列铅7中鉴定CYP和hERG清洁的mPGES-1抑制剂,进行了广泛的结构-活性关系(SAR)研究。在A,d和E形环的优化7得到许多有效的化合物,在160-950纳米范围内的人全血效价。选定的抑制剂21d,21j,21m,21n,21p和22b提供了对COX酶和mPGES-1亚型(mPGES-2和cPGES)的选择性,以及对前列腺素合酶的足够选择性。大多数测试的类似物证明在肝微粒体中具有所需的代谢稳定性,低的hERG和CYP耐受性。对多种化合物如大鼠,豚鼠,狗和食蟹猴讨论了先导化合物21j,21m和21p的口服药代动力学和生物利用度。此外,这些化合物还显示出对人孕烷X受体(hPXR)的低至中等活性。选定的引线21J进一步证明体内功效在急性痛觉过敏(ED 50和MIA诱导的骨关节炎疼痛模型(ED:39.6毫克/千克)50:106 mg / kg)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号