当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐pot Annulation for Biaryl‐fused Monocarba‐closo‐dodecaborate through Aromatic B−H Bond Disconnection
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-03-13 , DOI: 10.1002/asia.201800053 Gaku Akimoto 1, 2 , Mai Otsuka 1 , Kazunori Miyamoto 1 , Atsuya Muranaka 2 , Daisuke Hashizume 3 , Ryo Takita 2 , Masanobu Uchiyama 1, 2
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-03-13 , DOI: 10.1002/asia.201800053 Gaku Akimoto 1, 2 , Mai Otsuka 1 , Kazunori Miyamoto 1 , Atsuya Muranaka 2 , Daisuke Hashizume 3 , Ryo Takita 2 , Masanobu Uchiyama 1, 2
Affiliation
|
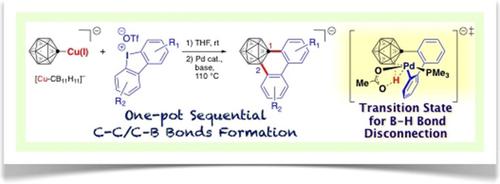
We have developed a one‐pot annulation reaction of monocarba‐closo‐dodecaborate with cyclic diaryliodonium salts to afford biaryl‐fused derivatives. Aryl functionalities are introduced at both the 1‐carbon and unreactive ortho‐boron vertices of the “σ‐aromatic” carborane cage without the need for pre‐functionalization. DFT calculations revealed that the palladium‐catalyzed C−B bond‐formation step in this process proceeds through a concerted metalation–deprotonation (CMD)‐type pathway for the B−H bond disconnection on the aromatic cage, though such bonds are generally regarded as hydridic.
中文翻译:

通过芳族B-H键断开对联芳基稠合一氨基甲酸酯-双十二羰基化合物的一锅法
我们已经开发monocarba-的一锅环反应闭合碳-dodecaborate与环二芳基碘盐,得到联芳融合衍生物。无需预功能化,即可在“σ-芳族”碳硼烷笼的1碳和非反应性邻硼顶点上引入芳基官能团。DFT计算表明,在此过程中,钯催化的C-B键形成步骤通过芳香族笼中B-H键断开的协同金属化-去质子化(CMD)型途径进行,尽管通常认为此类键为氢化的。
更新日期:2018-03-13
中文翻译:

通过芳族B-H键断开对联芳基稠合一氨基甲酸酯-双十二羰基化合物的一锅法
我们已经开发monocarba-的一锅环反应闭合碳-dodecaborate与环二芳基碘盐,得到联芳融合衍生物。无需预功能化,即可在“σ-芳族”碳硼烷笼的1碳和非反应性邻硼顶点上引入芳基官能团。DFT计算表明,在此过程中,钯催化的C-B键形成步骤通过芳香族笼中B-H键断开的协同金属化-去质子化(CMD)型途径进行,尽管通常认为此类键为氢化的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号