当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Substates of Ras To Interact with GAPs and Effectors: Revealed by Theoretical Simulations and FTIR Experiments
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpclett.8b00342 Yang Li 1, 2 , Yuwei Zhang 1 , Frederik Großerüschkamp 3 , Sara Stephan 3 , Qiang Cui 4 , Carsten Kötting 3 , Fei Xia 1, 5 , Klaus Gerwert 3
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpclett.8b00342 Yang Li 1, 2 , Yuwei Zhang 1 , Frederik Großerüschkamp 3 , Sara Stephan 3 , Qiang Cui 4 , Carsten Kötting 3 , Fei Xia 1, 5 , Klaus Gerwert 3
Affiliation
|
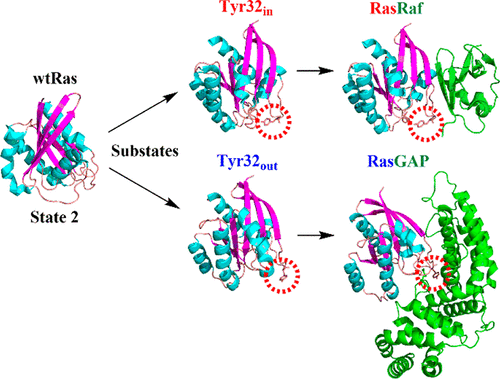
The oncogenic Ras protein adopts various specific conformational states to execute its function in signal transduction. The large number of Ras structures obtained from X-ray and NMR experiments illustrates the diverse conformations that Ras adopts. It is difficult, however, to connect specific structural features with Ras functions. We report the free-energy landscape of Ras·GTP based on extensive explicit solvent simulations. The free-energy map clearly shows that the functional state 2 of Ras·GTP in fact has two distinct substates, denoted here as “Tyr32in” and “Tyr32out”. Unbiased MD simulations show that the two substrates interconvert on the submicrosecond scale in solution, pointing to a novel mechanism for Ras·GTP to selectively interact with GAPs and effectors. This proposal is further supported by time-resolved FTIR experiments, which demonstrate that Tyr32 destabilizes the Ras·GAP complex and facilitates an efficient termination of Ras signaling.
中文翻译:

Ras 与 GAP 和效应器相互作用的特定亚状态:理论模拟和 FTIR 实验揭示
致癌Ras蛋白采用各种特定构象状态来执行其信号转导功能。 X射线和NMR实验获得的大量Ras结构说明了Ras采用的不同构象。然而,很难将特定的结构特征与 Ras 函数联系起来。我们基于广泛的显式溶剂模拟报告了 Ras·GTP 的自由能景观。自由能图清楚地表明 Ras·GTP 的功能状态 2 实际上具有两个不同的子状态,此处表示为“Tyr32 in ”和“Tyr32 out ”。无偏 MD 模拟表明,两种底物在溶液中以亚微秒级相互转化,指出 Ras·GTP 选择性与 GAP 和效应器相互作用的新机制。这一提议得到了时间分辨 FTIR 实验的进一步支持,该实验证明 Tyr32 使 Ras·GAP 复合物不稳定并促进 Ras 信号传导的有效终止。
更新日期:2018-02-28
中文翻译:

Ras 与 GAP 和效应器相互作用的特定亚状态:理论模拟和 FTIR 实验揭示
致癌Ras蛋白采用各种特定构象状态来执行其信号转导功能。 X射线和NMR实验获得的大量Ras结构说明了Ras采用的不同构象。然而,很难将特定的结构特征与 Ras 函数联系起来。我们基于广泛的显式溶剂模拟报告了 Ras·GTP 的自由能景观。自由能图清楚地表明 Ras·GTP 的功能状态 2 实际上具有两个不同的子状态,此处表示为“Tyr32 in ”和“Tyr32 out ”。无偏 MD 模拟表明,两种底物在溶液中以亚微秒级相互转化,指出 Ras·GTP 选择性与 GAP 和效应器相互作用的新机制。这一提议得到了时间分辨 FTIR 实验的进一步支持,该实验证明 Tyr32 使 Ras·GAP 复合物不稳定并促进 Ras 信号传导的有效终止。











































 京公网安备 11010802027423号
京公网安备 11010802027423号