Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-02-27 , DOI: 10.1016/j.bmc.2018.02.016 Jason M Rohde 1 , Kyle R Brimacombe 1 , Li Liu 1 , Michael E Pacold 2 , Adam Yasgar 1 , Dorian M Cheff 1 , Tobie D Lee 1 , Ganesha Rai 1 , Bolormaa Baljinnyam 1 , Zhuyin Li 1 , Anton Simeonov 1 , Matthew D Hall 1 , Min Shen 1 , David M Sabatini 3 , Matthew B Boxer 1
|
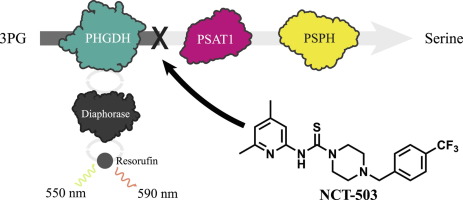
Proliferating cells, including cancer cells, obtain serine both exogenously and via the metabolism of glucose. By catalyzing the first, rate-limiting step in the synthesis of serine from glucose, phosphoglycerate dehydrogenase (PHGDH) controls flux through the biosynthetic pathway for this important amino acid and represents a putative target in oncology. To discover inhibitors of PHGDH, a coupled biochemical assay was developed and optimized to enable high-throughput screening for inhibitors of human PHGDH. Feedback inhibition was minimized by coupling PHGDH activity to two downstream enzymes (PSAT1 and PSPH), providing a marked improvement in enzymatic turnover. Further coupling of NADH to a diaphorase/resazurin system enabled a red-shifted detection readout, minimizing interference due to compound autofluorescence. With this protocol, over 400,000 small molecules were screened for PHGDH inhibition, and following hit validation and triage work, a piperazine-1-thiourea was identified. Following rounds of medicinal chemistry and SAR exploration, two probes (NCT-502 and NCT-503) were identified. These molecules demonstrated improved target activity and encouraging ADME properties, enabling in vitro assessment of the biological importance of PHGDH, and its role in the fate of serine in PHGDH-dependent cancer cells. This manuscript reports the assay development and medicinal chemistry leading to the development of NCT-502 and -503 reported in Pacold et al. (2016).
中文翻译:

发现和优化基于哌嗪-1-硫脲的人磷酸甘油酯脱氢酶抑制剂。
包括癌细胞在内的增殖细胞都通过葡萄糖代谢和外源获得丝氨酸。通过催化从葡萄糖合成丝氨酸的第一个限速步骤,磷酸甘油酸脱氢酶(PHGDH)控制了该重要氨基酸通过生物合成途径的通量,并代表了肿瘤学中的一个假定靶标。为了发现PHGDH的抑制剂,开发了一种偶联生化测定法并对其进行了优化,以能够高通量筛选人PHGDH的抑制剂。通过将PHGDH活性与两种下游酶(PSAT1和PSPH)偶联,可以将反馈抑制作用降至最低,从而显着改善酶的转化率。将NADH与心肌黄递酶/刃天青系统进一步偶联,可实现红移检测读数,从而将化合物自发荧光引起的干扰降至最低。使用此协议,超过400个,筛选了000个小分子的PHGDH抑制作用,并在命中验证和分类工作之后,鉴定出了哌嗪-1-硫脲。经过几轮药物化学和SAR探索,鉴定了两种探针(NCT-502和NCT-503)。这些分子表现出改善的靶标活性并具有ADME特性,从而使对PHGDH的生物学重要性及其在丝氨酸命运中的作用的体外评估,PHGDH依赖性癌细胞。该手稿报告了Pacold等人报道的导致NCT-502和-503发展的测定方法开发和药物化学。(2016)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号