当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A “Seleno Effect” Differentiates the Roles of Redox Active Cysteine Residues in Plasmodium falciparum Thioredoxin Reductase
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00004 John P. O’Keefe 1 , Christopher M. Dustin 1 , Drew Barber 1 , Gregg W. Snider 1 , Robert J. Hondal 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00004 John P. O’Keefe 1 , Christopher M. Dustin 1 , Drew Barber 1 , Gregg W. Snider 1 , Robert J. Hondal 1
Affiliation
|
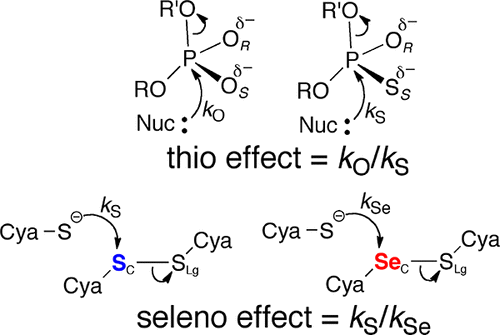
Here, we introduce the concept of the “seleno effect” in the study of oxidoreductases that catalyze thiol/disulfide exchange reactions. In these reactions, selenium can replace sulfur as a nucleophile, electrophile, or leaving group, and the resulting change in rate (the seleno effect) is defined as kS/kSe. In solution, selenium accelerates the rate of thiol/disulfide exchange regardless of its chemical role (e.g., nucleophile or electrophile). Here we show that this is not the case for enzyme catalyzed reactions and that the magnitude of the seleno effect can differentiate the role of each sulfur atom of a disulfide bond between that of an electrophile or leaving group. We used selenium for sulfur substitution to study the thiol/disulfide exchange step that occurs between the N-terminal redox center and the C-terminal disulfide-containing β-hairpin motif of Plasmodium falciparum thioredoxin reductase (PfTrxR), which has the sequence Gly-Cys535-Gly-Gly-Gly-Lys-Cys540-Gly. We assayed a truncated PfTrxR enzyme missing this C-terminal tail for disulfide-reductase activity using synthetic peptide substrates in which either Cys535 or Cys540 was replaced with selenocysteine (Sec). The results show that substitution of Cys535 with Sec resulted in a nearly 9-fold decrease in the rate of reduction, while substitution of Cys540 resulted in a 1.5-fold increase in the rate of reduction. We also produced full-length, semisynthetic enzymes in which Sec replaced either of these two Cys residues and observed similar results using E. coli thioredoxin as the substrate. In this assay, the observed seleno effect (kS/kSe) for the C535U mutant was 7.4, and that for the C540U mutant was 0.2.
中文翻译:

“ Seleno效应”区分了恶性疟原虫硫氧还蛋白还原酶中氧化还原活性半胱氨酸残基的作用。
在此,我们在催化硫醇/二硫键交换反应的氧化还原酶的研究中引入“硒代效应”的概念。在这些反应中,硒可以取代硫成为亲核试剂,亲电试剂或离去基团,并且由此产生的速率变化(硒代反应)定义为k S / k Se。在溶液中,硒可加速硫醇/二硫键的交换速率,而不管其化学作用(例如亲核试剂或亲电试剂)如何。在这里,我们表明酶催化反应不是这种情况,并且硒代作用的大小可以区分亲电子基团或离去基团之间二硫键的每个硫原子的作用。我们使用硒进行硫取代来研究恶性疟原虫硫氧还蛋白还原酶(PfTrxR)的N端氧化还原中心和C端含C端二硫键的β-发夹基序之间发生的巯基/二硫键交换步骤,该序列具有Gly- Cys 535 -Gly-Gly-Gly-Lys-Cys 540-Gly。我们使用合成肽底物(其中Cys 535或Cys 540替换为硒代半胱氨酸(Sec))分析了缺失此C末端尾部的截短的PfTrxR酶的二硫键还原酶活性。结果表明,用Sec取代Cys 535导致还原速率降低了近9倍,而用Cys 540取代导致还原速率提高了1.5倍。我们还产生了全长的半合成酶,其中Sec取代了这两个Cys残基中的任何一个,并使用大肠杆菌硫氧还蛋白作为底物观察到了相似的结果。在此测定法中,观察到的硒效应(k S / k Se)的C535U突变体为7.4,而C540U突变体为0.2。
更新日期:2018-02-27
中文翻译:

“ Seleno效应”区分了恶性疟原虫硫氧还蛋白还原酶中氧化还原活性半胱氨酸残基的作用。
在此,我们在催化硫醇/二硫键交换反应的氧化还原酶的研究中引入“硒代效应”的概念。在这些反应中,硒可以取代硫成为亲核试剂,亲电试剂或离去基团,并且由此产生的速率变化(硒代反应)定义为k S / k Se。在溶液中,硒可加速硫醇/二硫键的交换速率,而不管其化学作用(例如亲核试剂或亲电试剂)如何。在这里,我们表明酶催化反应不是这种情况,并且硒代作用的大小可以区分亲电子基团或离去基团之间二硫键的每个硫原子的作用。我们使用硒进行硫取代来研究恶性疟原虫硫氧还蛋白还原酶(PfTrxR)的N端氧化还原中心和C端含C端二硫键的β-发夹基序之间发生的巯基/二硫键交换步骤,该序列具有Gly- Cys 535 -Gly-Gly-Gly-Lys-Cys 540-Gly。我们使用合成肽底物(其中Cys 535或Cys 540替换为硒代半胱氨酸(Sec))分析了缺失此C末端尾部的截短的PfTrxR酶的二硫键还原酶活性。结果表明,用Sec取代Cys 535导致还原速率降低了近9倍,而用Cys 540取代导致还原速率提高了1.5倍。我们还产生了全长的半合成酶,其中Sec取代了这两个Cys残基中的任何一个,并使用大肠杆菌硫氧还蛋白作为底物观察到了相似的结果。在此测定法中,观察到的硒效应(k S / k Se)的C535U突变体为7.4,而C540U突变体为0.2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号