当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stepwise Hydrogen Atom and Proton Transfers in Dioxygen Reduction by Aryl-Alcohol Oxidase
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00106 Juan Carro 1 , Patricia Ferreira 2 , Angel T. Martínez 1 , Giovanni Gadda 3
Biochemistry ( IF 2.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.biochem.8b00106 Juan Carro 1 , Patricia Ferreira 2 , Angel T. Martínez 1 , Giovanni Gadda 3
Affiliation
|
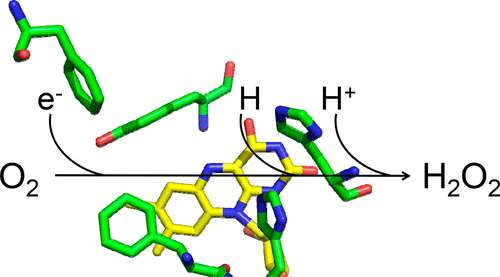
The mechanism of dioxygen reduction by the flavoenzyme aryl-alcohol oxidase was investigated with kinetic isotope, viscosity, and pL (pH/pD) effects in rapid kinetics experiments by stopped-flow spectrophotometry of the oxidative half-reaction of the enzyme. Double mixing of the enzyme in a stopped-flow spectrophotometer with [α-2H2]-p-methoxybenzyl alcohol and oxygen at varying aging times established a slow rate constant of 0.0023 s–1 for the wash-out of the D atom from the N5 atom of the reduced flavin. Thus, the deuterated substrate could be used to probe the cleavage of the N–H bond of the reduced flavin in the oxidative half-reaction. A significant and pH-independent substrate kinetic isotope effect (KIE) of 1.5 between pH 5.0 and 8.0 demonstrated that H transfer is partially limiting the oxidative half-reaction of the enzyme; a negligible solvent KIE of 1.0 between pD 5.0 and 8.0 proved a fast H+ transfer reaction that does not contribute to determining the flavin oxidation rates. Thus, a mechanism for dioxygen reduction in which the H atom originating from the reduced flavin and a H+ from a solvent exchangeable site are transferred in separate kinetic steps is proposed. The spectroscopic and kinetic data presented also showed a lack of stabilization of transient flavin intermediates. The substantial differences in the mechanistic details of O2 reduction by aryl-alcohol oxidase with respect to other alcohol oxidases like choline oxidase, pyranose 2-oxidase, and glucose oxidase further demonstrate the high level of versatility of the flavin cofactor in flavoenzymes.
中文翻译:

芳醇氧化酶在双氧还原中的逐步氢原子和质子转移
在快速动力学实验中,通过停止流式分光光度法对黄酮酶芳基-醇氧化酶的双氧还原机理进行了动力学同位素,粘度和pL(pH / pD)效应的研究,研究了该酶的氧化半反应。在停止流动的分光光度计中,将酶与[α- 2 H 2 ]-对甲氧基苄醇和氧气在不同的老化时间下双重混合,建立了0.0023 s –1的慢速常数用于从还原的黄素的N5原子中洗去D原子。因此,氘化的底物可用于探测在氧化半反应中还原的黄素的NH键的断裂。pH在5.0和8.0之间的显着且与pH无关的底物动力学同位素效应(KIE)为1.5,这表明H转移部分限制了该酶的氧化半反应。pD 5.0和8.0之间可忽略不计的溶剂KIE为1.0,证明了快速的H +转移反应,对确定黄素的氧化速率没有帮助。因此,一种双氧还原的机制,其中H原子源自还原的黄素和H +提出了将来自溶剂可交换位点的化合物在单独的动力学步骤中转移。所提供的光谱和动力学数据还显示出瞬态黄素中间体缺乏稳定性。相对于其他醇氧化酶(如胆碱氧化酶,吡喃糖2-氧化酶和葡萄糖氧化酶),芳基醇氧化酶还原O 2的机理细节上的实质差异进一步证明了黄素辅因子在黄素酶中具有高水平的多功能性。
更新日期:2018-02-27
中文翻译:

芳醇氧化酶在双氧还原中的逐步氢原子和质子转移
在快速动力学实验中,通过停止流式分光光度法对黄酮酶芳基-醇氧化酶的双氧还原机理进行了动力学同位素,粘度和pL(pH / pD)效应的研究,研究了该酶的氧化半反应。在停止流动的分光光度计中,将酶与[α- 2 H 2 ]-对甲氧基苄醇和氧气在不同的老化时间下双重混合,建立了0.0023 s –1的慢速常数用于从还原的黄素的N5原子中洗去D原子。因此,氘化的底物可用于探测在氧化半反应中还原的黄素的NH键的断裂。pH在5.0和8.0之间的显着且与pH无关的底物动力学同位素效应(KIE)为1.5,这表明H转移部分限制了该酶的氧化半反应。pD 5.0和8.0之间可忽略不计的溶剂KIE为1.0,证明了快速的H +转移反应,对确定黄素的氧化速率没有帮助。因此,一种双氧还原的机制,其中H原子源自还原的黄素和H +提出了将来自溶剂可交换位点的化合物在单独的动力学步骤中转移。所提供的光谱和动力学数据还显示出瞬态黄素中间体缺乏稳定性。相对于其他醇氧化酶(如胆碱氧化酶,吡喃糖2-氧化酶和葡萄糖氧化酶),芳基醇氧化酶还原O 2的机理细节上的实质差异进一步证明了黄素辅因子在黄素酶中具有高水平的多功能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号