当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Synthesis of H2O2 on AgPt Octahedra: The Importance of Ag–Pt Coordination for High H2O2 Selectivity
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acscatal.7b04186 Neil M. Wilson 1 , Yung-Tin Pan 1 , Yu-Tsun Shao 2 , Jian-Min Zuo 2 , Hong Yang 1 , David W. Flaherty 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acscatal.7b04186 Neil M. Wilson 1 , Yung-Tin Pan 1 , Yu-Tsun Shao 2 , Jian-Min Zuo 2 , Hong Yang 1 , David W. Flaherty 1
Affiliation
|
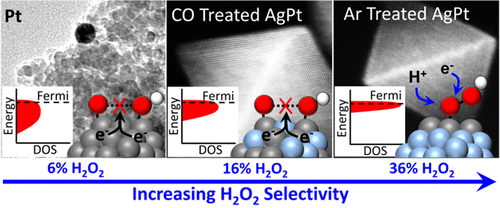
H2O2 production by direct synthesis (H2 + O2 → H2O2) is a promising alternative to the energy-intensive anthraquinone oxidation process and to the use of chlorine for oxidation chemistry. Steady-state H2O2 selectivities are approximately 10-fold greater on AgPt octahedra (50%) than on Pt nanoparticles of similar size (6%). Moreover, the initial H2O2 formation rates and selectivities are sensitive to the fractional coverage of Pt atoms and their location on the surfaces of AgPt octahedra, which can be controlled by exposing these catalysts to either CO or inert gases at 373 K to produce Pt-rich (16% initial H2O2 selectivity) or Pt-poor (36% initial H2O2 selectivity) surfaces. Increasing the coordination of Pt to Ag significantly modifies the electronic structure of Pt active sites, which is reflected by a shift in the ν(C=O) singleton frequency in 13CO from 2016 cm–1 on Pt to ∼1975 cm–1 on AgPt. These bimetallic AgPt catalysts present lower activation enthalpies (ΔH⧧) for H2O2 formation (29 kJ mol–1 on Pt to 5 kJ mol–1 on AgPt) but a lesser decrease for H2O formation (26 kJ mol–1 on Pt to 16 kJ mol–1 on AgPt). Comparisons of H2O2 selectivities, ΔH⧧ values, and differences among the 13CO singleton frequencies show that a combination of coordinating Ag to Pt and inducing strain modifies the electronic structure of individual Pt atoms, causing them to bind η1-species (e.g., CO) more strongly than on Pt nanoparticles. Yet the dramatic increase in the number of isolated Pt atoms increases H2O2 selectivities by decreasing the number of Pt atom ensembles of sufficient size to cleave O–O bonds and form H2O.
中文翻译:

在AgPt八面体上直接合成H 2 O 2:Ag-Pt配位对高H 2 O 2选择性的重要性
ħ 2 ö 2生产通过直接合成(H 2 + O 2 →H 2 ö 2)是一种很有前途的替代能源密集型蒽醌氧化过程,并使用氯的氧化化学。在AgPt八面体(50%)上,稳态H 2 O 2选择性大约是在类似大小的Pt纳米颗粒上(6%)的10倍。此外,初始H 2 O 2形成速率和选择性对Pt原子的分数覆盖及其在AgPt八面体表面上的位置很敏感,可以通过将这些催化剂暴露于373 K的CO或惰性气体中以生成富Pt(初始H为16%)来控制2 O 2选择性)或贫铂(初始H 2 O 2选择性为36%)表面。增加Pt与Ag的配位会显着改变Pt活性位的电子结构,这反映在13 CO中ν(C = O)单峰频率从Pt的2016 cm –1转变为1975年的1975 cm –1。 AgPt。这些双金属催化剂AgPt本较低的活化焓(Δ ħ⧧)用于h 2 ö 2形成(29千焦摩尔-1上的Pt到为5kJ摩尔-1上AgPt),但为H A较小减少2澳组(26千焦摩尔-1在Pt至16千焦耳摩尔-1上AgPt )。h的比较2和ö 2选择性,Δ ħ ⧧值,并且之间的差异13 CO单频率表明,配位的Ag向Pt和诱发应变修改个人的Pt原子的电子结构,引起的组合他们结合η 1物种(例如,CO)比Pt纳米颗粒更强。然而,通过减少足够大小的Pt原子团的数量来裂解O-O键并形成H 2 O ,孤立的Pt原子数量的急剧增加增加了H 2 O 2的选择性。
更新日期:2018-02-27
中文翻译:

在AgPt八面体上直接合成H 2 O 2:Ag-Pt配位对高H 2 O 2选择性的重要性
ħ 2 ö 2生产通过直接合成(H 2 + O 2 →H 2 ö 2)是一种很有前途的替代能源密集型蒽醌氧化过程,并使用氯的氧化化学。在AgPt八面体(50%)上,稳态H 2 O 2选择性大约是在类似大小的Pt纳米颗粒上(6%)的10倍。此外,初始H 2 O 2形成速率和选择性对Pt原子的分数覆盖及其在AgPt八面体表面上的位置很敏感,可以通过将这些催化剂暴露于373 K的CO或惰性气体中以生成富Pt(初始H为16%)来控制2 O 2选择性)或贫铂(初始H 2 O 2选择性为36%)表面。增加Pt与Ag的配位会显着改变Pt活性位的电子结构,这反映在13 CO中ν(C = O)单峰频率从Pt的2016 cm –1转变为1975年的1975 cm –1。 AgPt。这些双金属催化剂AgPt本较低的活化焓(Δ ħ⧧)用于h 2 ö 2形成(29千焦摩尔-1上的Pt到为5kJ摩尔-1上AgPt),但为H A较小减少2澳组(26千焦摩尔-1在Pt至16千焦耳摩尔-1上AgPt )。h的比较2和ö 2选择性,Δ ħ ⧧值,并且之间的差异13 CO单频率表明,配位的Ag向Pt和诱发应变修改个人的Pt原子的电子结构,引起的组合他们结合η 1物种(例如,CO)比Pt纳米颗粒更强。然而,通过减少足够大小的Pt原子团的数量来裂解O-O键并形成H 2 O ,孤立的Pt原子数量的急剧增加增加了H 2 O 2的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号