当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydration Promoted by a Methylene Group: A Volumetric Study on Alkynes in Water
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.jpcb.8b00843 Satoshi Shibuta 1 , Hiroshi Imamura 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.jpcb.8b00843 Satoshi Shibuta 1 , Hiroshi Imamura 2
Affiliation
|
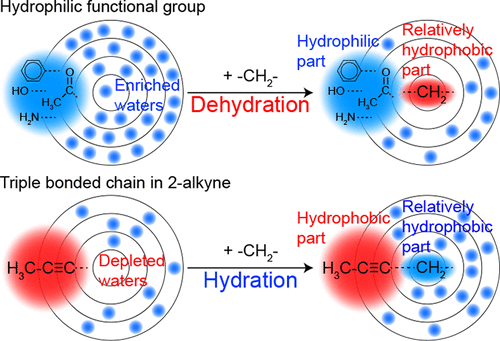
Hydrocarbons including a methylene group are generally considered a hydrophobic building block, in the sense that the density of their hydration water is lower than that of bulk water. However, is the methylene group always hydrophobic? In this study, we experimentally determined the partial molar volume of a methylene group in water as 14.01 ± 0.46 cm3 mol–1 for 1-alkyne, 9.83 ± 0.35 cm3 mol–1 for 2-alkyne, and 11.39 ± 0.55 cm3 mol–1 for 3-alkyne. These values are all unusually small compared to the ∼16 cm3 mol–1 for model compounds from the literature. The subsequent volumetric analysis on the basis of the Kirkwood–Buff parameter indicates that the hydration water is enriched by the addition of a methylene group for 2-alkyne, while it is depleted for the reported model compounds that contain hydrophilic functional groups, 1-alkyne, and 3-alkyne. Our findings suggest that the triple bonded carbons in 2-alkyne that reduce hydration water act as a hydrophobic group in 2-alkyne. Thus, the methylene group should be called “hydrophilic” in this case because it actually recovers the hydration water when placed next to more hydrophobic groups. Therefore, we conclude that the hydrophobicity of a methylene group varies depending on its hydration environment due to other functional groups in the solute.
中文翻译:

亚甲基基团促进水合作用:水中炔烃的体积研究
就其水合水的密度低于本体水的密度而言,包括亚甲基的烃通常被认为是疏水性结构单元。但是,亚甲基始终是疏水的吗?在这项研究中,我们通过实验确定了水中的亚甲基部分摩尔体积,对于1-炔为14.01±0.46 cm 3 mol –1,对于2-炔为9.83±0.35 cm 3 mol –1,以及11.39±0.55 cm 3 3-炔烃为mol –1。与〜16 cm 3 mol –1相比,这些值都异常小。用于文献中的模型化合物。随后的基于Kirkwood–Buff参数的体积分析表明,水合水通过添加2-炔基的亚甲基来富集,而对于报告的包含亲水性官能团的模型化合物1-炔基则被消耗掉了。和3-炔烃。我们的发现表明2-炔烃中减少水合水的三键碳在2-炔烃中充当疏水基团。因此,在这种情况下,亚甲基应称为“亲水性”,因为当它与疏水性更高的基团并排放置时,实际上可以回收水合水。因此,我们得出结论,由于溶质中的其他官能团,亚甲基的疏水性取决于其水合环境而变化。
更新日期:2018-02-27
中文翻译:

亚甲基基团促进水合作用:水中炔烃的体积研究
就其水合水的密度低于本体水的密度而言,包括亚甲基的烃通常被认为是疏水性结构单元。但是,亚甲基始终是疏水的吗?在这项研究中,我们通过实验确定了水中的亚甲基部分摩尔体积,对于1-炔为14.01±0.46 cm 3 mol –1,对于2-炔为9.83±0.35 cm 3 mol –1,以及11.39±0.55 cm 3 3-炔烃为mol –1。与〜16 cm 3 mol –1相比,这些值都异常小。用于文献中的模型化合物。随后的基于Kirkwood–Buff参数的体积分析表明,水合水通过添加2-炔基的亚甲基来富集,而对于报告的包含亲水性官能团的模型化合物1-炔基则被消耗掉了。和3-炔烃。我们的发现表明2-炔烃中减少水合水的三键碳在2-炔烃中充当疏水基团。因此,在这种情况下,亚甲基应称为“亲水性”,因为当它与疏水性更高的基团并排放置时,实际上可以回收水合水。因此,我们得出结论,由于溶质中的其他官能团,亚甲基的疏水性取决于其水合环境而变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号