当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Thermodynamic Analysis of the Solubility of Limonin in Eight Organic Solvents and Ethyl Acetate + 2-Propanol Binary Solvents from 283.2 to 323.2 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.jced.7b00851 Jie-Ping Fan , Tian-Tao Yuan , Jia-Xin Yu , Xue-Hong Zhang , Ya-Hui Cao
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1021/acs.jced.7b00851 Jie-Ping Fan , Tian-Tao Yuan , Jia-Xin Yu , Xue-Hong Zhang , Ya-Hui Cao
|
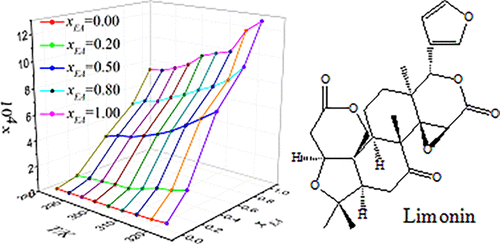
In the present study, the solubility of limonin in eight organic solvents and a binary solvent system (ethyl acetate + 2-propanol) was determined by the high-performance liquid chromatography analysis method within the temperature range from 283.2 to 323.2 K. The results showed that the solubility of limonin increased with temperature in the eight pure solvents and the binary solvent mixtures. In the binary solvent system ethyl acetate + 2-propanol, the solubility decreased with the initial mole fraction of ethyl acetate. The experimental solubility of limonin was well correlated with temperature by the simplified thermodynamic equation, modified Apelblat equation, λh equation, and NRTL equation. Furthermore, the solubility of limonin in the binary solvent mixtures was correlated with both temperature and initial solvent composition of the binary solvent mixtures by the Jouyban–Acree model, van’t Hoff–Jouyban–Acree model, modified Apelblat–Jouyban–Acree model, Ma model, Sun model, and NRTL model. Finally, the thermodynamic properties of the mixing process, including mixing Gibbs energy, mixing enthalpy, and mixing entropy, were also calculated.
中文翻译:

柠檬苦素在283.2至323.2 K范围内的8种有机溶剂和乙酸乙酯+ 2-丙二醇二元溶剂中的溶解度的测定和热力学分析
在本研究中,通过高效液相色谱分析方法在283.2至323.2 K的温度范围内测定了柠檬苦素在8种有机溶剂和二元溶剂体系(乙酸乙酯+ 2-丙醇)中的溶解度。结果表明柠檬苦素在八种纯溶剂和二元溶剂混合物中的溶解度随温度的升高而增加。在二元溶剂体系乙酸乙酯+ 2-丙醇中,溶解度随乙酸乙酯的初始摩尔分数而降低。通过简化的热力学方程,修正的Apelblat方程λh,柠檬苦素的实验溶解度与温度具有良好的相关性方程和NRTL方程。此外,通过Jouyban-Acree模型,van't Hoff-Jouyban-Acree模型,改良的Apelblat-Jouyban-Acree模型,柠檬苦素在二元溶剂混合物中的溶解度与二元溶剂混合物的温度和初始溶剂组成相关, Ma模型,Sun模型和NRTL模型。最后,还计算了混合过程的热力学性质,包括混合吉布斯能量,混合焓和混合熵。
更新日期:2018-02-27
中文翻译:

柠檬苦素在283.2至323.2 K范围内的8种有机溶剂和乙酸乙酯+ 2-丙二醇二元溶剂中的溶解度的测定和热力学分析
在本研究中,通过高效液相色谱分析方法在283.2至323.2 K的温度范围内测定了柠檬苦素在8种有机溶剂和二元溶剂体系(乙酸乙酯+ 2-丙醇)中的溶解度。结果表明柠檬苦素在八种纯溶剂和二元溶剂混合物中的溶解度随温度的升高而增加。在二元溶剂体系乙酸乙酯+ 2-丙醇中,溶解度随乙酸乙酯的初始摩尔分数而降低。通过简化的热力学方程,修正的Apelblat方程λh,柠檬苦素的实验溶解度与温度具有良好的相关性方程和NRTL方程。此外,通过Jouyban-Acree模型,van't Hoff-Jouyban-Acree模型,改良的Apelblat-Jouyban-Acree模型,柠檬苦素在二元溶剂混合物中的溶解度与二元溶剂混合物的温度和初始溶剂组成相关, Ma模型,Sun模型和NRTL模型。最后,还计算了混合过程的热力学性质,包括混合吉布斯能量,混合焓和混合熵。











































 京公网安备 11010802027423号
京公网安备 11010802027423号