当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Poly(ester)–poly(silyl methacrylate) copolymers: synthesis and hydrolytic degradation kinetics†
Polymer Chemistry ( IF 4.1 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c8py00052b Qingyi Xie 1, 2, 3, 4, 5 , Chunfeng Ma 1, 2, 3, 4 , Guangzhao Zhang 1, 2, 3, 4 , Christine Bressy 5, 6, 7, 8, 9
Polymer Chemistry ( IF 4.1 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c8py00052b Qingyi Xie 1, 2, 3, 4, 5 , Chunfeng Ma 1, 2, 3, 4 , Guangzhao Zhang 1, 2, 3, 4 , Christine Bressy 5, 6, 7, 8, 9
Affiliation
|
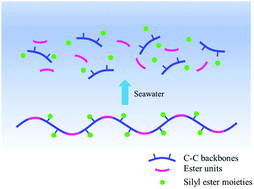
Copolymerization via a radical ring opening polymerization of 2-methylene-1,3-dioxepane (MDO) with various silyl methacrylates including bis(trimethylsiloxy)methylsilyl methacrylate (MATM2), triisopropylsilyl methacrylate (TIPSiMA) and tributylsilyl methacrylate (TBSiMA) has been reported. Kinetics of polymerization show that the monomers react in a near-linear manner during the early hours and the consumption of silyl monomers is faster than MDO. The silyl ester side groups in the copolymers are prone to hydrolysis on contact with artificial seawater (ASW), leading to a decrease of the water contact angle of the copolymer film with immersion time. The nature of the silyl ester groups was shown to be able to tune the hydrolysis rate of the copolymers and the hydrophilicity of polymer films. The hydrolysis rates of MATM2 and TBSiMA are faster than the TIPSiMA one. The water uptake test shows that copolymers containing MATM2 and TBSiMA have higher water absorption than the copolymer based on TIPSiMA. MDO copolymers with MATM2 and TBSiMA show a significant decline in molar mass by 90% and 86% after immersion in ASW at room temperature for 7 weeks, mainly due to the cleavage of main-chain ester bonds. Their degradation is accelerated at 60 °C; meanwhile the TIPSiMA-based polyester does not show notable degradation at both temperatures, indicating that the hydrolytic degradation rate of the polymer backbone is tuned by the amount of hydrolyzed ester side groups.
中文翻译:

聚(酯)-聚(甲基丙烯酸甲硅烷基酯)共聚物:合成和水解降解动力学†
通过共聚已经报道了2-亚甲基-1,3-二氧戊环(MDO)与各种甲基丙烯酸甲硅烷基酯的自由基开环聚合,所述甲基丙烯酸甲硅烷基酯包括甲基丙烯酸双(三甲基甲硅烷氧基)甲基甲硅烷基酯(MATM2),甲基丙烯酸三异丙基甲硅烷基酯(TIPSiMA)和甲基丙烯酸三丁基甲硅烷基酯(TBSiMA)。聚合反应动力学表明,在早期,单体以近线性方式反应,甲硅烷基单体的消耗比MDO快。共聚物中的甲硅烷基酯侧基在与人造海水(ASW)接触时易于水解,从而导致共聚物膜的水接触角随浸入时间而减小。已显示甲硅烷基酯基团的性质能够调节共聚物的水解速率和聚合物膜的亲水性。MATM2和TBSiMA的水解速率比TIPSiMA的水解速率快。吸水测试表明,含有MATM2和TBSiMA的共聚物比基于TIPSiMA的共聚物具有更高的吸水率。具有MATM2和TBSiMA的MDO共聚物在室温下在ASW中浸泡7周后,摩尔质量显着下降90%和86%,这主要是由于主链酯键的断裂。它们的降解在60°C时会加速;同时,基于TIPSiMA的聚酯在两个温度下均未显示出明显的降解,这表明聚合物主链的水解降解速率可通过水解酯侧基的数量来调节。具有MATM2和TBSiMA的MDO共聚物在室温下在ASW中浸泡7周后,摩尔质量显着下降90%和86%,这主要是由于主链酯键的断裂。它们的降解在60°C时会加速;同时,基于TIPSiMA的聚酯在两个温度下均未显示出明显的降解,这表明聚合物主链的水解降解速率可通过水解酯侧基的数量来调节。具有MATM2和TBSiMA的MDO共聚物在室温下在ASW中浸泡7周后,摩尔质量显着下降90%和86%,这主要是由于主链酯键的断裂。它们的降解在60°C时会加速;同时,基于TIPSiMA的聚酯在两个温度下均未显示出明显的降解,这表明聚合物主链的水解降解速率可通过水解酯侧基的数量来调节。
更新日期:2018-02-27
中文翻译:

聚(酯)-聚(甲基丙烯酸甲硅烷基酯)共聚物:合成和水解降解动力学†
通过共聚已经报道了2-亚甲基-1,3-二氧戊环(MDO)与各种甲基丙烯酸甲硅烷基酯的自由基开环聚合,所述甲基丙烯酸甲硅烷基酯包括甲基丙烯酸双(三甲基甲硅烷氧基)甲基甲硅烷基酯(MATM2),甲基丙烯酸三异丙基甲硅烷基酯(TIPSiMA)和甲基丙烯酸三丁基甲硅烷基酯(TBSiMA)。聚合反应动力学表明,在早期,单体以近线性方式反应,甲硅烷基单体的消耗比MDO快。共聚物中的甲硅烷基酯侧基在与人造海水(ASW)接触时易于水解,从而导致共聚物膜的水接触角随浸入时间而减小。已显示甲硅烷基酯基团的性质能够调节共聚物的水解速率和聚合物膜的亲水性。MATM2和TBSiMA的水解速率比TIPSiMA的水解速率快。吸水测试表明,含有MATM2和TBSiMA的共聚物比基于TIPSiMA的共聚物具有更高的吸水率。具有MATM2和TBSiMA的MDO共聚物在室温下在ASW中浸泡7周后,摩尔质量显着下降90%和86%,这主要是由于主链酯键的断裂。它们的降解在60°C时会加速;同时,基于TIPSiMA的聚酯在两个温度下均未显示出明显的降解,这表明聚合物主链的水解降解速率可通过水解酯侧基的数量来调节。具有MATM2和TBSiMA的MDO共聚物在室温下在ASW中浸泡7周后,摩尔质量显着下降90%和86%,这主要是由于主链酯键的断裂。它们的降解在60°C时会加速;同时,基于TIPSiMA的聚酯在两个温度下均未显示出明显的降解,这表明聚合物主链的水解降解速率可通过水解酯侧基的数量来调节。具有MATM2和TBSiMA的MDO共聚物在室温下在ASW中浸泡7周后,摩尔质量显着下降90%和86%,这主要是由于主链酯键的断裂。它们的降解在60°C时会加速;同时,基于TIPSiMA的聚酯在两个温度下均未显示出明显的降解,这表明聚合物主链的水解降解速率可通过水解酯侧基的数量来调节。









































 京公网安备 11010802027423号
京公网安备 11010802027423号