当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular stabilization of a catalytic [FeFe]-hydrogenase mimic investigated by experiment and theory†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c7dt04837h Indresh Kumar Pandey 1, 2, 3, 4 , Mookan Natarajan 1, 2, 3, 4 , Hemlata Faujdar 1, 2, 3, 4 , Firasat Hussain 1, 2, 3, 4 , Matthias Stein 5, 6, 7, 8 , Sandeep Kaur-Ghumaan 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c7dt04837h Indresh Kumar Pandey 1, 2, 3, 4 , Mookan Natarajan 1, 2, 3, 4 , Hemlata Faujdar 1, 2, 3, 4 , Firasat Hussain 1, 2, 3, 4 , Matthias Stein 5, 6, 7, 8 , Sandeep Kaur-Ghumaan 1, 2, 3, 4, 5
Affiliation
|
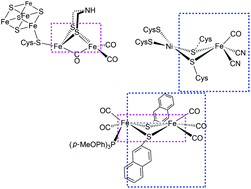
The mono-substituted complex [Fe2(CO)5(μ-naphthalene-2-thiolate)2(P(PhOMe-p)3)] was prepared taking after the structural principles from both [NiFe] and [FeFe]-hydrogenase enzymes. Crystal structures are reported for this complex and the all carbonyl analogue. The bridging naphthalene thiolates resemble μ-bridging cysteine amino acids. One of the naphthyl moieties forms π–π stacking interactions with the terminal bulky phosphine ligand in the crystal structure and in calculations. This interaction stabilizes the reduced and protonated forms during electrocatalytic proton reduction in the presence of acetic acid and hinders the rotation of the phosphine ligand. The intramolecular π–π stabilization, the electrochemistry and the mechanism of the hydrogen evolution reaction were investigated using computational approaches.
中文翻译:

通过实验和理论研究了催化[FeFe]-加氢酶模拟物的分子内稳定性†
单取代的复合物[Fe 2(CO)5(μ-萘-2-硫酸酯)2(P(PhOMe- p)3)]是根据[NiFe]和[FeFe]氢化酶的结构原理制备的。据报道该络合物和所有羰基类似物的晶体结构。桥接的萘酚硫醇盐类似于μ-桥接的半胱氨酸氨基酸。萘基之一与晶体结构和计算中的末端庞大的膦配体形成π-π堆积相互作用。这种相互作用使在乙酸存在下的电催化质子还原过程中的还原形式和质子化形式稳定,并阻碍了膦配体的旋转。使用计算方法研究了分子内π-π的稳定性,电化学和析氢反应的机理。
更新日期:2018-02-27
中文翻译:

通过实验和理论研究了催化[FeFe]-加氢酶模拟物的分子内稳定性†
单取代的复合物[Fe 2(CO)5(μ-萘-2-硫酸酯)2(P(PhOMe- p)3)]是根据[NiFe]和[FeFe]氢化酶的结构原理制备的。据报道该络合物和所有羰基类似物的晶体结构。桥接的萘酚硫醇盐类似于μ-桥接的半胱氨酸氨基酸。萘基之一与晶体结构和计算中的末端庞大的膦配体形成π-π堆积相互作用。这种相互作用使在乙酸存在下的电催化质子还原过程中的还原形式和质子化形式稳定,并阻碍了膦配体的旋转。使用计算方法研究了分子内π-π的稳定性,电化学和析氢反应的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号