当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactive force-field molecular dynamics study on graphene oxide reinforced cement composite: functional group de-protonation, interfacial bonding and strengthening mechanism†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c8cp00006a Dongshuai Hou 1, 2, 3 , Tiejun Yang 1, 2, 3 , Jinhui Tang 3, 4, 5, 6 , Shaochun Li 1, 2, 3
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-27 00:00:00 , DOI: 10.1039/c8cp00006a Dongshuai Hou 1, 2, 3 , Tiejun Yang 1, 2, 3 , Jinhui Tang 3, 4, 5, 6 , Shaochun Li 1, 2, 3
Affiliation
|
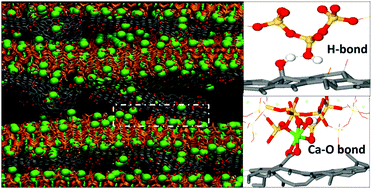
Graphene oxide (GO) reinforced cement nanocomposites open up a new path for sustainable concrete design. In this paper, reactive force-field molecular dynamics was utilized to investigate the structure, reactivity and interfacial bonding of calcium silicate hydrate (C–S–H)/GO nanocomposite functionalized by hydroxyl (C–OH), epoxy (C–O–C), carboxyl (COOH) and sulfonic (SO3H) groups with a coverage of 10%. The silicate chains in the hydrophilic C–S–H substrate provided numerous non-bridging oxygen sites and counter ions (Ca ions) with high reactivity, which allowed interlayer water molecules to dissociate into Si–OH and Ca–OH. On the other hand, protons dissociated from the functional groups and transferred to non-bridging sites in C–S–H, producing carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and Si–OH. The de-protonation degree of the different groups in the vicinity of the C–S–H surface was in the following order: COOH (SO3H) > C–OH > C–O–C. In the GO–COOH sheet, most COOH groups were de-protonated to COO− groups, which enhanced the polarity and hydrophilicity of the GO sheets and formed stable COOCa bonds with neighboring Ca ions. The de-protonated COO− could also accept H bonds from Si–OH in the C–S–H gel, which further strengthened the interfacial connection. On the contrary, in the GO–Oo sheet, only 8% of the epoxy group was stretched open by the Ca ions and transformed to carbonyl group, showing weak polarity and connection with the C–S–H sheet. Furthermore, uniaxial tensile test on different C–S–H/GO models revealed that C–S–H reinforced with GO–COOH and GO–OH had better interfacial cohesive strength and ductility than that observed under tensile loading. Under the reaction force field, the dissociation of water, the proton exchange between the C–S–H and GO structure, and Oc–Ca–Os bond breakage occurred to resist tensile loading. The weakest mechanical behavior observed in the G/C–S–H, GO–Oo/C–S–H and GO–SO3H/C–S–H composites was attributed to the poor bonding, dissociation of functional groups and instability of atoms in the interface region. Hopefully, the molecular-scale strengthening mechanisms could provide a scientific guide for sustainable design of cement composites.
O) and Si–OH. The de-protonation degree of the different groups in the vicinity of the C–S–H surface was in the following order: COOH (SO3H) > C–OH > C–O–C. In the GO–COOH sheet, most COOH groups were de-protonated to COO− groups, which enhanced the polarity and hydrophilicity of the GO sheets and formed stable COOCa bonds with neighboring Ca ions. The de-protonated COO− could also accept H bonds from Si–OH in the C–S–H gel, which further strengthened the interfacial connection. On the contrary, in the GO–Oo sheet, only 8% of the epoxy group was stretched open by the Ca ions and transformed to carbonyl group, showing weak polarity and connection with the C–S–H sheet. Furthermore, uniaxial tensile test on different C–S–H/GO models revealed that C–S–H reinforced with GO–COOH and GO–OH had better interfacial cohesive strength and ductility than that observed under tensile loading. Under the reaction force field, the dissociation of water, the proton exchange between the C–S–H and GO structure, and Oc–Ca–Os bond breakage occurred to resist tensile loading. The weakest mechanical behavior observed in the G/C–S–H, GO–Oo/C–S–H and GO–SO3H/C–S–H composites was attributed to the poor bonding, dissociation of functional groups and instability of atoms in the interface region. Hopefully, the molecular-scale strengthening mechanisms could provide a scientific guide for sustainable design of cement composites.
中文翻译:

氧化石墨烯增强水泥复合材料的反应力场分子动力学研究:官能团去质子化,界面结合和增强机理†
氧化石墨烯(GO)增强的水泥纳米复合材料为可持续的混凝土设计开辟了一条新途径。在本文中,利用反应力场分子动力学研究了被羟基(C–OH),环氧(C–O–)官能化的水合硅酸钙(C–S–H)/ GO纳米复合材料的结构,反应性和界面键合。 C),羧基(COOH)和磺酸基(SO 3 H),覆盖率为10%。亲水性C–H–H底物中的硅酸盐链提供了许多高活性的非桥接氧位点和抗衡离子(Ca离子),这使层间水分子解离为Si–OH和Ca–OH。另一方面,质子从官能团上解离并转移到C–H中的非桥接位点,产生羰基(C![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O)和Si–OH。C–S–H表面附近不同基团的去质子化程度按以下顺序排列:COOH(SO 3 H)> C–OH> C–OC。在GO-COOH片,最COOH基团被去质子化,COO -基团,其增强了GO片材和形成稳定COOCa键的极性和亲水性与邻近Ca离子。去质子化COO -也可以在C–S–H凝胶中接受来自Si–OH的H键,这进一步加强了界面连接。相反,在GO–Oo片中,只有8%的环氧基被Ca离子张开并转变为羰基,显示出弱极性并与C–S–H片连接。此外,在不同的C–S–H / GO模型上进行的单轴拉伸试验表明,用GO–COOH和GO–OH增强的C–S–H具有比在拉伸载荷下观察到的更好的界面粘结强度和延展性。在反作用力场下,水的解离,C–S–H和GO结构之间的质子交换以及Oc–Ca–Os的键断裂发生,从而抵抗了拉伸载荷。在G / C–S–H,GO–Oo / C–S–H和GO–SO 3中观察到的最弱的机械行为H / C–S–H复合材料归因于不良的键合,官能团的解离和界面区域中原子的不稳定性。希望分子尺度增强机制可以为水泥复合材料的可持续设计提供科学指导。
O)和Si–OH。C–S–H表面附近不同基团的去质子化程度按以下顺序排列:COOH(SO 3 H)> C–OH> C–OC。在GO-COOH片,最COOH基团被去质子化,COO -基团,其增强了GO片材和形成稳定COOCa键的极性和亲水性与邻近Ca离子。去质子化COO -也可以在C–S–H凝胶中接受来自Si–OH的H键,这进一步加强了界面连接。相反,在GO–Oo片中,只有8%的环氧基被Ca离子张开并转变为羰基,显示出弱极性并与C–S–H片连接。此外,在不同的C–S–H / GO模型上进行的单轴拉伸试验表明,用GO–COOH和GO–OH增强的C–S–H具有比在拉伸载荷下观察到的更好的界面粘结强度和延展性。在反作用力场下,水的解离,C–S–H和GO结构之间的质子交换以及Oc–Ca–Os的键断裂发生,从而抵抗了拉伸载荷。在G / C–S–H,GO–Oo / C–S–H和GO–SO 3中观察到的最弱的机械行为H / C–S–H复合材料归因于不良的键合,官能团的解离和界面区域中原子的不稳定性。希望分子尺度增强机制可以为水泥复合材料的可持续设计提供科学指导。
更新日期:2018-02-27
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and Si–OH. The de-protonation degree of the different groups in the vicinity of the C–S–H surface was in the following order: COOH (SO3H) > C–OH > C–O–C. In the GO–COOH sheet, most COOH groups were de-protonated to COO− groups, which enhanced the polarity and hydrophilicity of the GO sheets and formed stable COOCa bonds with neighboring Ca ions. The de-protonated COO− could also accept H bonds from Si–OH in the C–S–H gel, which further strengthened the interfacial connection. On the contrary, in the GO–Oo sheet, only 8% of the epoxy group was stretched open by the Ca ions and transformed to carbonyl group, showing weak polarity and connection with the C–S–H sheet. Furthermore, uniaxial tensile test on different C–S–H/GO models revealed that C–S–H reinforced with GO–COOH and GO–OH had better interfacial cohesive strength and ductility than that observed under tensile loading. Under the reaction force field, the dissociation of water, the proton exchange between the C–S–H and GO structure, and Oc–Ca–Os bond breakage occurred to resist tensile loading. The weakest mechanical behavior observed in the G/C–S–H, GO–Oo/C–S–H and GO–SO3H/C–S–H composites was attributed to the poor bonding, dissociation of functional groups and instability of atoms in the interface region. Hopefully, the molecular-scale strengthening mechanisms could provide a scientific guide for sustainable design of cement composites.
O) and Si–OH. The de-protonation degree of the different groups in the vicinity of the C–S–H surface was in the following order: COOH (SO3H) > C–OH > C–O–C. In the GO–COOH sheet, most COOH groups were de-protonated to COO− groups, which enhanced the polarity and hydrophilicity of the GO sheets and formed stable COOCa bonds with neighboring Ca ions. The de-protonated COO− could also accept H bonds from Si–OH in the C–S–H gel, which further strengthened the interfacial connection. On the contrary, in the GO–Oo sheet, only 8% of the epoxy group was stretched open by the Ca ions and transformed to carbonyl group, showing weak polarity and connection with the C–S–H sheet. Furthermore, uniaxial tensile test on different C–S–H/GO models revealed that C–S–H reinforced with GO–COOH and GO–OH had better interfacial cohesive strength and ductility than that observed under tensile loading. Under the reaction force field, the dissociation of water, the proton exchange between the C–S–H and GO structure, and Oc–Ca–Os bond breakage occurred to resist tensile loading. The weakest mechanical behavior observed in the G/C–S–H, GO–Oo/C–S–H and GO–SO3H/C–S–H composites was attributed to the poor bonding, dissociation of functional groups and instability of atoms in the interface region. Hopefully, the molecular-scale strengthening mechanisms could provide a scientific guide for sustainable design of cement composites.
中文翻译:

氧化石墨烯增强水泥复合材料的反应力场分子动力学研究:官能团去质子化,界面结合和增强机理†
氧化石墨烯(GO)增强的水泥纳米复合材料为可持续的混凝土设计开辟了一条新途径。在本文中,利用反应力场分子动力学研究了被羟基(C–OH),环氧(C–O–)官能化的水合硅酸钙(C–S–H)/ GO纳米复合材料的结构,反应性和界面键合。 C),羧基(COOH)和磺酸基(SO 3 H),覆盖率为10%。亲水性C–H–H底物中的硅酸盐链提供了许多高活性的非桥接氧位点和抗衡离子(Ca离子),这使层间水分子解离为Si–OH和Ca–OH。另一方面,质子从官能团上解离并转移到C–H中的非桥接位点,产生羰基(C
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O)和Si–OH。C–S–H表面附近不同基团的去质子化程度按以下顺序排列:COOH(SO 3 H)> C–OH> C–OC。在GO-COOH片,最COOH基团被去质子化,COO -基团,其增强了GO片材和形成稳定COOCa键的极性和亲水性与邻近Ca离子。去质子化COO -也可以在C–S–H凝胶中接受来自Si–OH的H键,这进一步加强了界面连接。相反,在GO–Oo片中,只有8%的环氧基被Ca离子张开并转变为羰基,显示出弱极性并与C–S–H片连接。此外,在不同的C–S–H / GO模型上进行的单轴拉伸试验表明,用GO–COOH和GO–OH增强的C–S–H具有比在拉伸载荷下观察到的更好的界面粘结强度和延展性。在反作用力场下,水的解离,C–S–H和GO结构之间的质子交换以及Oc–Ca–Os的键断裂发生,从而抵抗了拉伸载荷。在G / C–S–H,GO–Oo / C–S–H和GO–SO 3中观察到的最弱的机械行为H / C–S–H复合材料归因于不良的键合,官能团的解离和界面区域中原子的不稳定性。希望分子尺度增强机制可以为水泥复合材料的可持续设计提供科学指导。
O)和Si–OH。C–S–H表面附近不同基团的去质子化程度按以下顺序排列:COOH(SO 3 H)> C–OH> C–OC。在GO-COOH片,最COOH基团被去质子化,COO -基团,其增强了GO片材和形成稳定COOCa键的极性和亲水性与邻近Ca离子。去质子化COO -也可以在C–S–H凝胶中接受来自Si–OH的H键,这进一步加强了界面连接。相反,在GO–Oo片中,只有8%的环氧基被Ca离子张开并转变为羰基,显示出弱极性并与C–S–H片连接。此外,在不同的C–S–H / GO模型上进行的单轴拉伸试验表明,用GO–COOH和GO–OH增强的C–S–H具有比在拉伸载荷下观察到的更好的界面粘结强度和延展性。在反作用力场下,水的解离,C–S–H和GO结构之间的质子交换以及Oc–Ca–Os的键断裂发生,从而抵抗了拉伸载荷。在G / C–S–H,GO–Oo / C–S–H和GO–SO 3中观察到的最弱的机械行为H / C–S–H复合材料归因于不良的键合,官能团的解离和界面区域中原子的不稳定性。希望分子尺度增强机制可以为水泥复合材料的可持续设计提供科学指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号