Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-02-27 , DOI: 10.1016/j.bioorg.2018.02.012 Adejoke N. Kolawole , Valentine T. Akinladejo , Olusola O. Elekofehinti , Afolabi C. Akinmoladun , Ayodele O. Kolawole
|
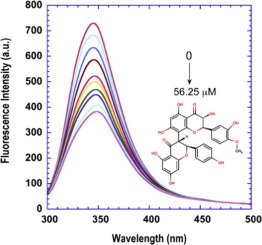
Aldehyde dehydrogenases (ALDHs) are a diverse family of enzymes that catalyze the NAD(P)+-dependent detoxification of toxic aldehyde compounds. ALDHs are also involved in non-enzymatic ligand binding to endobiotics and xenobiotics. Here, the enzyme crucial non-canonical and non-catalytic interaction with kolaflavanone, a component of kolaviron, and a major bioflavonoid isolated from Garcinia kola (Bitter kola) was characterized by various spectroscopic and in silico approaches under simulated physiological condition. Kolaflavanone quenched the intrinsic fluorescence of ALDH in a concentration dependent manner with an effective quenching constant (Ksv) of 1.14 × 103 L.mol−1 at 25 °C. The enzyme has one binding site for kolaflavanone with a binding constant (Ka) of 2.57 × 104 L.mol−1 and effective Forster resonance energy transfer (FRET) of 4.87 nm. The bonding process was enthalpically driven. The reaction was not spontaneous and was predominantly characterized by Van der Waals forces and hydrogen bond. The flavonoid bonding slightly perturbed the secondary and tertiary structures of ALDH that was ‘tryptophan-gated’. The interaction was regulated by both diffusion and ionic strength. Molecular docking showed the binding of kolaflavanone was at the active site of ALDH and the participation of some amino acid residues in the complex formation with −9.6 kcal mol−1 binding energy. The profiles of atomic fluctuations indicated the rigidity of the ligand-binding site during the simulation. With these, ALDH as a subtle nano-particle determinant of kolaviron bioavailability and efficacy is hereby proposed.
中文翻译:

科拉维酮-可乐黄酮与醛脱氢酶相互作用的实验和计算模型
醛脱氢酶(ALDHs)是催化NAD(P)+依赖性的有毒醛类化合物解毒作用的多种酶。ALDHs还参与与内源性和异源性生物素的非酶配体结合。在此,在模拟的生理条件下,通过各种光谱和计算机模拟的方法,对与从茄子可乐中分离出的主要生物类黄酮(可乐黄酮的成分)和主要生物类黄酮进行了至关重要的非规范和非催化相互作用。Kolaflavanone以浓度依赖的方式猝灭ALDH的内在荧光,其有效猝灭常数(K sv)为1.14×10 3 L.mol -1在25°C下。该酶具有一个与可乐酮的结合位点,结合常数(K a)为2.57×10 4 L.mol -1,有效的Forster共振能量转移(FRET)为4.87 nm。粘合过程是由焓驱动的。反应不是自发的,主要以范德华力和氢键为特征。类黄酮键轻微干扰了“色氨酸门控”的ALDH的二级和三级结构。相互作用受扩散和离子强度的调节。分子对接表明可乐黄酮的结合在ALDH的活性位点处,并且某些氨基酸残基参与了-9.6 kcal mol -1的复合物的形成。结合能。原子涨落曲线表明了模拟过程中配体结合位点的刚性。通过这些,提出了ALDH作为Kolaviron生物利用度和功效的微妙的纳米颗粒决定因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号