Journal of the American Society for Mass Spectrometry ( IF 3.1 ) Pub Date : 2018-02-27 , DOI: 10.1007/s13361-017-1884-8 William D. Blincoe 1 , Agustina Rodriguez-Granillo 2 , Josep Saurí 1 , Nicholas A. Pierson 1 , Leo A. Joyce 1 , Ian Mangion 1 , Huaming Sheng 1
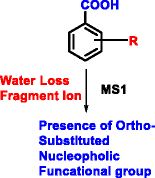
Benzoic acid/ester/amide derivatives are common moieties in pharmaceutical compounds and present a challenge in positional isomer identification by traditional tandem mass spectrometric analysis. A method is presented for exploiting the gas-phase neighboring group participation (NGP) effect to differentiate ortho-substituted benzoic acid/ester derivatives with high resolution mass spectrometry (HRMS1). Significant water/alcohol loss (>30% abundance in MS1 spectra) was observed for ortho-substituted nucleophilic groups; these fragment peaks are not observable for the corresponding para and meta-substituted analogs. Experiments were also extended to the analysis of two intermediates in the synthesis of suvorexant (Belsomra) with additional analysis conducted with nuclear magnetic resonance (NMR), density functional theory (DFT), and ion mobility spectrometry-mass spectrometry (IMS-MS) studies. Significant water/alcohol loss was also observed for 1-substituted 1, 2, 3-triazoles but not for the isomeric 2-substituted 1, 2, 3-triazole analogs. IMS-MS, NMR, and DFT studies were conducted to show that the preferred orientation of the 2-substituted triazole rotamer was away from the electrophilic center of the reaction, whereas the 1-subtituted triazole was oriented in close proximity to the center. Abundance of NGP product was determined to be a product of three factors: (1) proton affinity of the nucleophilic group; (2) steric impact of the nucleophile; and (3) proximity of the nucleophile to carboxylic acid/ester functional groups.
ᅟ
中文翻译:

鉴定
苯甲酸/酯/酰胺衍生物是药物化合物中的常见部分,并且通过传统的串联质谱分析对位置异构体鉴定提出了挑战。提出了一种利用气相邻近基团参与(NGP)效应以高分辨率质谱(HRMS 1)区分邻位取代的苯甲酸/酯衍生物的方法。对于邻位取代的亲核基团,观察到明显的水/醇损失(在MS 1光谱中> 30%的丰度);这些片段的峰对于相应的对位和元不可见-取代的类似物。实验还扩展到了suvorexant(Belsomra)合成中的两种中间体的分析,并通过核磁共振(NMR),密度泛函理论(DFT)和离子迁移谱-质谱(IMS-MS)研究进行了其他分析。 。对于1-取代的1、2、3-三唑,还观察到明显的水/醇损失,但对于异构的2-取代的1、2、3-三唑类似物没有观察到。进行IMS-MS,NMR和DFT研究以表明2-取代的三唑旋转异构体的优选取向远离反应的亲电子中心,而1-取代的三唑的取向紧邻该中心。确定NGP产物的丰度是三个因素的产物:(1)亲核基团的质子亲和力;(2)亲核基团的质子亲和力。(2)亲核试剂的空间影响;(3)亲核试剂与羧酸/酯官能团的接近度。

ᅟ











































 京公网安备 11010802027423号
京公网安备 11010802027423号