当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of (Cu, S) Co‐doped SnO2 for Electrocatalytic Reduction of CO2 to Formate at Low Overpotential
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-03-09 , DOI: 10.1002/celc.201800104 Xueyan Hu 1 , Huimin Yang 1 , Minmin Guo 1 , Mengting Gao 1 , Erhui Zhang 1 , Haoyang Tian 1 , Zhenhai Liang 1 , Xian Liu 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-03-09 , DOI: 10.1002/celc.201800104 Xueyan Hu 1 , Huimin Yang 1 , Minmin Guo 1 , Mengting Gao 1 , Erhui Zhang 1 , Haoyang Tian 1 , Zhenhai Liang 1 , Xian Liu 2
Affiliation
|
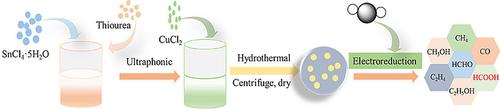
A novel copper (Cu) and sulfur (S) co‐doped SnO2 material prepared by a facile hydrothermal method is demonstrated as an efficient electrocatalyst for the reduction of CO2 to formate. The as‐prepared SC10 holds rutile structure, whereas both Cu and S are doped well into the SnO2, in which S2− and Cu2+ replace O2− and Sn4+, respectively. The overpotential observed in CO2‐saturated 0.5 M NaHCO3 solution is as low as 130 mV (vs. RHE) at −0.75 V (vs. Ag/AgCl) and the maximum current density also increases to 5.5 mA cm−2 at −1.2 V, which is 7‐times higher than pure SnO2. The catalyst is stable for more than 33 h and the Faradic efficiency of formate is 58.5 %. The as‐synthesized catalyst will open up a novel route for efficient reduction to formate and has a great potential for relieving the greenhouse effect.
中文翻译:

(Cu,S)共掺杂SnO2的合成和表征,用于在低超电势下将CO2电催化还原成甲酸酯
通过简便的水热法制备的新型铜(Cu)和硫(S)共掺杂的SnO 2材料被证明是将CO 2还原为甲酸的有效电催化剂。制成的SC 10具有金红石结构,而Cu和S都很好地掺杂到SnO 2中,其中S 2-和Cu 2+分别代替O 2-和Sn 4+。在-0.75 V(vs. Ag / AgCl)下,在充满CO 2的0.5 M NaHCO 3溶液中观察到的过电势低至130 mV(vs. RHE),最大电流密度也增加到5.5 mA cm -2在−1.2 V下,比纯SnO 2高7倍。催化剂稳定超过33小时,甲酸的法拉第效率为58.5%。合成后的催化剂将开辟一条有效还原甲酸酯的新途径,并具有减轻温室效应的巨大潜力。
更新日期:2018-03-09
中文翻译:

(Cu,S)共掺杂SnO2的合成和表征,用于在低超电势下将CO2电催化还原成甲酸酯
通过简便的水热法制备的新型铜(Cu)和硫(S)共掺杂的SnO 2材料被证明是将CO 2还原为甲酸的有效电催化剂。制成的SC 10具有金红石结构,而Cu和S都很好地掺杂到SnO 2中,其中S 2-和Cu 2+分别代替O 2-和Sn 4+。在-0.75 V(vs. Ag / AgCl)下,在充满CO 2的0.5 M NaHCO 3溶液中观察到的过电势低至130 mV(vs. RHE),最大电流密度也增加到5.5 mA cm -2在−1.2 V下,比纯SnO 2高7倍。催化剂稳定超过33小时,甲酸的法拉第效率为58.5%。合成后的催化剂将开辟一条有效还原甲酸酯的新途径,并具有减轻温室效应的巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号