Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-02-27 , DOI: 10.1016/j.jinorgbio.2018.02.022 Haotian Lei , Bruce E. Bowler
|
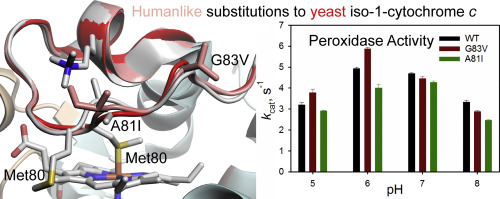
Structural studies of yeast iso-1-cytochrome c (L.J. McClelland, T.-C. Mou, M.E. Jeakins-Cooley, S.R. Sprang, B.E. Bowler, Proc. Natl. Acad. Sci. U.S.A. 111 (2014) 6648–6653) show that modest movement of Ω-loop D (residues 70–85, average RMSD versus the native structure: 0.81 Å) permits loss of Met80-heme ligation creating an available coordination site to catalyze the peroxidase activity mediated by cytochrome c early in apoptosis. However, Ala81 and Gly83 move significantly (RMSDs of 2.18 and 1.26 Å, respectively). Ala81 and Gly83 evolve to Ile and Val, respectively, in human cytochrome c and peroxidase activity decreases 25-fold relative to the yeast protein at pH 7. To test the hypothesis that these residues evolved to restrict the peroxidase activity of cytochrome c, A81I and G83V variants of yeast iso-1-cytochrome c were prepared. For both variants, the apparent pKa of the alkaline transition increases by 0.2 to 0.3 relative to the wild type (WT) protein and the rate of opening the heme crevice is slowed. The cooperativity of acid unfolding is decreased for the G83V variant. At pH 7 and 8, the catalytic rate constant, kcat, for the peroxidase activity of both variants decreases relative to WT, consistent with the effects on alkaline isomerization. Below pH 7, the loss in the cooperativity of acid unfolding causes kcat for peroxidase activity to increase for the G83V variant relative to WT. Neither variant decreases kcat to the level of the human protein, indicating that other residues also contribute to the low peroxidase activity of human cytochrome c.
中文翻译:

酵母异-1-细胞色素c的Ω环D的类人取代仅适度影响动力学和过氧化物酶活性
酵母异-1-细胞色素c的结构研究(LJ McClelland,T.-C。Mou,ME Jeakins-Cooley,SR Sprang,BE Bowler,美国国家科学院学报111(2014)6648–6653)显示Ω环D的适度运动(残基70-85,平均RMSD与天然结构:0.81Å)允许Met80-血红素连接的丧失,从而形成一个可用的配位位点,从而在细胞凋亡的早期催化细胞色素c介导的过氧化物酶活性。但是,Ala81和Gly83会明显移动(RMSD分别为2.18和1.26Å)。在人类细胞色素c中,Ala81和Gly83分别进化为Ile和Val相对于pH 7的酵母蛋白,过氧化物酶的活性降低了25倍。为了检验这些残基进化为限制细胞色素c的过氧化物酶活性的假说,制备了酵母异-1-细胞色素c的A81I和G83V变体。对于这两个变体,相对于野生型(WT)蛋白,碱性转变的表观p K a增加0.2至0.3,并且打开血红素缝隙的速率减慢。对于G83V变体,酸解折叠的协同作用降低。在pH 7和8下,催化速率常数k cat,因为两个变体的过氧化物酶活性相对于野生型降低,这与对碱性异构化的作用一致。在pH低于7时,酸解折叠的协同性的损失导致相对于野生型,G83V变体的过氧化物酶活性的k cat增加。两种变体均未将k cat降低至人类蛋白质水平,表明其他残基也导致人类细胞色素c的过氧化物酶活性低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号