当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inversion of Configuration at the Phosphorus Nucleophile in the Diastereoselective and Enantioselective Synthesis of P‐Stereogenic syn‐Phosphiranes from Chiral Epoxides
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801427 Jake A. Muldoon 1 , Balázs R. Varga 1 , Meaghan M. Deegan 1 , Timothy W. Chapp 1 , Ádám M. Eördögh 1 , Russell P. Hughes 1 , David S. Glueck 1 , Curtis E. Moore 2 , Arnold L. Rheingold 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801427 Jake A. Muldoon 1 , Balázs R. Varga 1 , Meaghan M. Deegan 1 , Timothy W. Chapp 1 , Ádám M. Eördögh 1 , Russell P. Hughes 1 , David S. Glueck 1 , Curtis E. Moore 2 , Arnold L. Rheingold 2
Affiliation
|
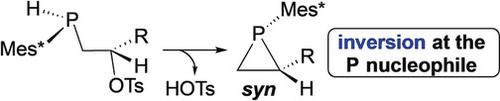
Nucleophilic substitution results in inversion of configuration at the electrophilic carbon center (SN2) or racemization (SN1). The stereochemistry of the nucleophile is rarely considered, but phosphines, which have a high barrier to pyramidal inversion, attack electrophiles with retention of configuration at P. Surprisingly, cyclization of bifunctional secondary phosphine alkyl tosylates proceeded under mild conditions with inversion of configuration at the nucleophile to yield P‐stereogenic syn‐phosphiranes. DFT studies suggested that the novel stereochemistry results from acid‐promoted tosylate dissociation to yield an intermediate phosphenium‐bridged cation, which undergoes syn‐selective cyclization.
中文翻译:

从手性环氧树脂对映异构对映体和对映体选择性合成对映体中磷亲核体的构型
亲核取代导致亲电子碳中心(S N 2)或外消旋化(S N 1)的构型反转。亲核试剂的立体化学很少考虑,但是对金字塔倒置具有高阻隔性的膦会攻击亲电试剂并保留P的构型。令人惊讶的是,双功能仲膦烷基甲苯磺酸盐的环化反应在温和的条件下进行,亲核试剂的构型反转以产生P-立体顺-phosphiranes。DFT研究表明,新的立体化学是由酸促进的甲苯磺酸酯解离产生中间体yield桥联的阳离子,该阳离子经历了顺选择性环化。
更新日期:2018-03-23
中文翻译:

从手性环氧树脂对映异构对映体和对映体选择性合成对映体中磷亲核体的构型
亲核取代导致亲电子碳中心(S N 2)或外消旋化(S N 1)的构型反转。亲核试剂的立体化学很少考虑,但是对金字塔倒置具有高阻隔性的膦会攻击亲电试剂并保留P的构型。令人惊讶的是,双功能仲膦烷基甲苯磺酸盐的环化反应在温和的条件下进行,亲核试剂的构型反转以产生P-立体顺-phosphiranes。DFT研究表明,新的立体化学是由酸促进的甲苯磺酸酯解离产生中间体yield桥联的阳离子,该阳离子经历了顺选择性环化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号