当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Tambromycin by Combining Chemocatalytic and Biocatalytic C−H Functionalization
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801165 Xiao Zhang 1 , Emma King-Smith 1 , Hans Renata 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801165 Xiao Zhang 1 , Emma King-Smith 1 , Hans Renata 1
Affiliation
|
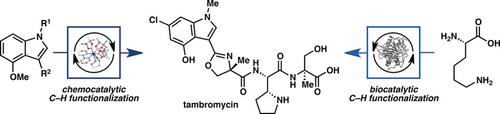
A combination of genomic and metabolomic approaches recently resulted in the identification of a nonribosomal tetrapeptide tambromycin, which possesses promising antiproliferative activity and several unusual structural features, including a densely substituted indole, a methyloxazoline ring, and an unusual pyrrolidine‐containing amino acid called tambroline. In this work, we identify a concise synthetic route to access tambromycin, which relies on the strategic use of biocatalytic and chemocatalytic C−H functionalization methods to prepare two key precursors to the natural product in an efficient and scalable manner. The success of our study highlights the benefits of applying the principles of biocatalytic retrosynthesis as well as C−H functionalization logic to the synthesis of complex molecular scaffolds.
中文翻译:

结合化学催化和生物催化CH官能团的全合成坦布霉素
基因组学和代谢组学方法的结合最近导致鉴定出一种非核糖体四肽坦布霉素,它具有有希望的抗增殖活性和几种不同寻常的结构特征,包括稠密取代的吲哚,甲基恶唑啉环和一种不寻常的含有吡咯烷的氨基酸,称为坦布罗林。在这项工作中,我们确定了一条简捷的合成坦布霉素合成途径,该途径依赖于对生物催化和化学催化CH功能化方法的战略性使用,以高效且可扩展的方式制备天然产物的两种关键前体。我们研究的成功凸显了将生物催化逆合成原理以及CH功能化逻辑应用于复杂分子支架合成的好处。
更新日期:2018-03-23
中文翻译:

结合化学催化和生物催化CH官能团的全合成坦布霉素
基因组学和代谢组学方法的结合最近导致鉴定出一种非核糖体四肽坦布霉素,它具有有希望的抗增殖活性和几种不同寻常的结构特征,包括稠密取代的吲哚,甲基恶唑啉环和一种不寻常的含有吡咯烷的氨基酸,称为坦布罗林。在这项工作中,我们确定了一条简捷的合成坦布霉素合成途径,该途径依赖于对生物催化和化学催化CH功能化方法的战略性使用,以高效且可扩展的方式制备天然产物的两种关键前体。我们研究的成功凸显了将生物催化逆合成原理以及CH功能化逻辑应用于复杂分子支架合成的好处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号