当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distinctive Stress-Stiffening Responses of Regenerated Silk Fibroin Protein Polymers under Nanoscale Gap Geometries: Effect of Shear on Silk Fibroin-Based Materials
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.biomac.8b00070 Yuanzhong Zhang 1 , Yuchen Zuo 1 , Shihao Wen 1 , Yupeng Hu 1 , Younjin Min 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.biomac.8b00070 Yuanzhong Zhang 1 , Yuchen Zuo 1 , Shihao Wen 1 , Yupeng Hu 1 , Younjin Min 1
Affiliation
|
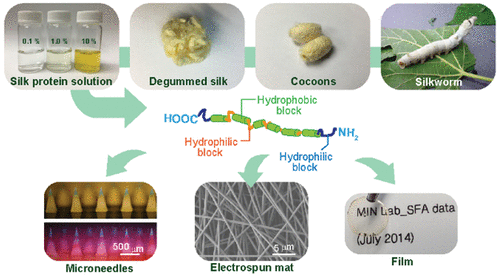
Interfacial dynamics, assembly processes, and changes in nanostructures and mechanical properties of Bombyx mori silk fibroin (SF) proteins under varying degrees of nanoconfinement without and with lateral shear are investigated. When only compressive confinement forces were applied, SF proteins adsorbed on the surfaces experienced conformational changes following the Alexander-de Gennes theory of polymer brushes. By contrast, when SF proteins were exposed to a simultaneous nanoconfinement and shear, remarkable changes in interaction forces were observed, displaying the second order phase transitions, which are attributed to the formation of SF micelles and globular superstructural aggregates via hierarchical assembly processes. The resultant nanostructured SF aggregates show several folds greater elastic moduli than those of SF films prepared by drop-casting and compression-only and even degummed SF fibers. Such a striking improvement in mechanical strength is ascribed to a directional organization of β-sheet nanocrystals, effectively driven by nanoconfinement and shear stress-induced stiffing and ordering mechanisms.
中文翻译:

纳米级间隙几何结构下再生丝素蛋白蛋白聚合物的独特应力-刚度响应:剪切对丝素蛋白基材料的影响
家蚕的界面动力学,组装过程以及纳米结构和力学性能的变化丝素蛋白(SF)蛋白质在不同程度的纳米约束下有无横向剪切和横向剪切。当仅施加压缩限制力时,吸附在表面上的SF蛋白会遵循Alexander-de Gennes聚合物刷理论,发生构象变化。相比之下,当SF蛋白同时受到纳米约束和剪切作用时,观察到相互作用力发生显着变化,显示出二级相变,这归因于SF胶束和球状超结构聚集体通过分层组装过程形成。所得的纳米结构SF聚集体的弹性模量比通过滴铸和仅压缩甚至脱胶的SF纤维制备的SF膜的弹性模量高出几倍。
更新日期:2018-02-26
中文翻译:

纳米级间隙几何结构下再生丝素蛋白蛋白聚合物的独特应力-刚度响应:剪切对丝素蛋白基材料的影响
家蚕的界面动力学,组装过程以及纳米结构和力学性能的变化丝素蛋白(SF)蛋白质在不同程度的纳米约束下有无横向剪切和横向剪切。当仅施加压缩限制力时,吸附在表面上的SF蛋白会遵循Alexander-de Gennes聚合物刷理论,发生构象变化。相比之下,当SF蛋白同时受到纳米约束和剪切作用时,观察到相互作用力发生显着变化,显示出二级相变,这归因于SF胶束和球状超结构聚集体通过分层组装过程形成。所得的纳米结构SF聚集体的弹性模量比通过滴铸和仅压缩甚至脱胶的SF纤维制备的SF膜的弹性模量高出几倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号