当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chlorinated Ethene Reactivity with Vitamin B12 Is Governed by Cobalamin Chloroethylcarbanions as Crossroads of Competing Pathways
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acscatal.7b02945 Benjamin Heckel 1, 2 , Kristopher McNeill 3 , Martin Elsner 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acscatal.7b02945 Benjamin Heckel 1, 2 , Kristopher McNeill 3 , Martin Elsner 1, 2
Affiliation
|
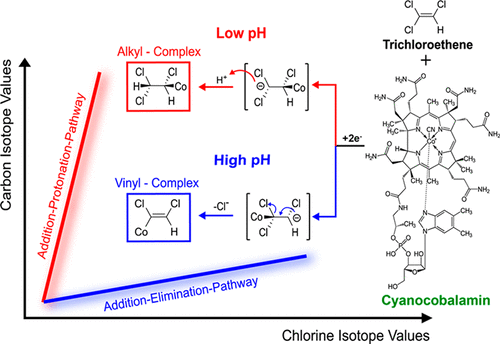
Chlorinated ethenes are toxic groundwater contaminants. Although they can be dechlorinated by microorganisms, reductive dehalogenases, and their corrinoid cofactor, biochemical reaction mechanisms remain unsolved. This study uncovers a mechanistic shift revealed by contrasting compound-specific carbon (ε13C) and chlorine (ε37Cl) isotope effects between perchloroethene, PCE (ε37Cl = −4.0‰) and cis-dichloroethene, cis-DCE (ε37Cl = −1.5‰), and a pH-dependent shift for trichloroethene, TCE (from ε37Cl = −5.2‰ at pH 12 to ε37Cl = −1.2‰ at pH 5). Different pathways are supported also by pH-dependent reaction rates, TCE product distribution, and hydrogen isotope effects. Mass balance deficits revealed reversible and irreversible cobalamin-substrate association, whereas high-resolution mass spectrometry narrowed down possible structures to chloroalkyl and chlorovinyl cobalamin complexes. Combined experimental evidence is inconsistent with initial electron transfer or alkyl or vinyl complexes as shared intermediates of both pathways. In contrast, it supports cobalamin chlorocarbanions as key intermediates from which Cl– elimination produces vinyl complexes (explaining rates and products of TCE at high pH), whereas protonation generates less reactive alkyl complexes (explaining rates and products of TCE at low pH). Multielement isotope effect analysis holds promise to identify these competing mechanisms also in real dehalogenases, microorganisms, and even contaminated aquifers.
中文翻译:

维生素B 12的氯化乙烯反应性由钴胺素氯乙基碳阴离子控制,是竞争途径的十字路口
氯化乙烯是有毒的地下水污染物。尽管它们可以被微生物,还原性脱卤化酶及其类皮质激素辅因子脱氯,但生化反应机制仍未解决。本研究揭示一种机械换档揭示通过对比特定化合物-碳(ε 13 C)和氯(ε 37 Cl)的perchloroethene之间的同位素效应,PCE(ε 37 CL = -4.0‰)和顺式-dichloroethene,顺-DCE(ε 37 CL = -1.5‰),和pH依赖性移三氯乙烯,三氯乙烯(从ε 37 CL = -5.2‰在pH 12至ε 37在pH 5时Cl = -1.2‰)。pH依赖的反应速率,TCE产物分布和氢同位素效应也支持不同的途径。质量平衡缺陷显示钴胺素与底物的可逆和不可逆结合,而高分辨率质谱法将可能的结构缩小为氯烷基和氯乙烯基钴胺素复合物。综合的实验证据与初始电子转移或烷基或乙烯基络合物作为两种途径的共同中间体不一致。相比之下,它支持钴胺素氯碳阴离子作为关键中间体,而Cl –消除会生成乙烯基络合物(在高pH下说明TCE的速率和产物),而质子化反应生成的烷基络合物较少(在低pH值下说明TCE的速率和产物)。多元素同位素效应分析有望在真实的脱卤酶,微生物甚至受污染的含水层中识别出这些竞争机制。
更新日期:2018-02-26
中文翻译:

维生素B 12的氯化乙烯反应性由钴胺素氯乙基碳阴离子控制,是竞争途径的十字路口
氯化乙烯是有毒的地下水污染物。尽管它们可以被微生物,还原性脱卤化酶及其类皮质激素辅因子脱氯,但生化反应机制仍未解决。本研究揭示一种机械换档揭示通过对比特定化合物-碳(ε 13 C)和氯(ε 37 Cl)的perchloroethene之间的同位素效应,PCE(ε 37 CL = -4.0‰)和顺式-dichloroethene,顺-DCE(ε 37 CL = -1.5‰),和pH依赖性移三氯乙烯,三氯乙烯(从ε 37 CL = -5.2‰在pH 12至ε 37在pH 5时Cl = -1.2‰)。pH依赖的反应速率,TCE产物分布和氢同位素效应也支持不同的途径。质量平衡缺陷显示钴胺素与底物的可逆和不可逆结合,而高分辨率质谱法将可能的结构缩小为氯烷基和氯乙烯基钴胺素复合物。综合的实验证据与初始电子转移或烷基或乙烯基络合物作为两种途径的共同中间体不一致。相比之下,它支持钴胺素氯碳阴离子作为关键中间体,而Cl –消除会生成乙烯基络合物(在高pH下说明TCE的速率和产物),而质子化反应生成的烷基络合物较少(在低pH值下说明TCE的速率和产物)。多元素同位素效应分析有望在真实的脱卤酶,微生物甚至受污染的含水层中识别出这些竞争机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号