当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Functional Studies of a Pyran Synthase Domain from a trans-Acyltransferase Assembly Line
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acschembio.8b00049 Drew T. Wagner 1 , Zhicheng Zhang 2 , Roy A. Meoded 3 , Alexis J. Cepeda 1 , Jörn Piel 3 , Adrian T. Keatinge-Clay 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acschembio.8b00049 Drew T. Wagner 1 , Zhicheng Zhang 2 , Roy A. Meoded 3 , Alexis J. Cepeda 1 , Jörn Piel 3 , Adrian T. Keatinge-Clay 1, 2
Affiliation
|
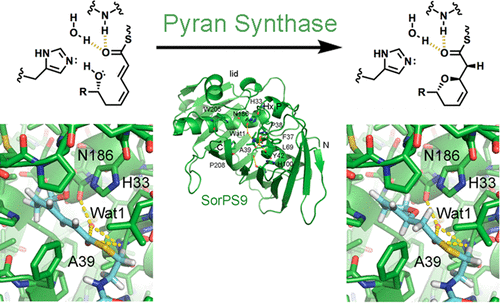
trans-Acyltransferase assembly lines possess enzymatic domains often not observed in their better characterized cis-acyltransferase counterparts. Within this repertoire of largely unexplored biosynthetic machinery is a class of enzymes called the pyran synthases that catalyze the formation of five- and six-membered cyclic ethers from diverse polyketide chains. The 1.55 Å resolution crystal structure of a pyran synthase domain excised from the ninth module of the sorangicin assembly line highlights the similarity of this enzyme to the ubiquitous dehydratase domain and provides insight into the mechanism of ring formation. Functional assays of point mutants reveal the central importance of the active site histidine that is shared with the dehydratases as well as the supporting role of a neighboring semiconserved asparagine.
中文翻译:

反式酰基转移酶组装线的吡喃合酶结构域的结构和功能研究
反式-酰基转移酶装配线具有通常在其更好表征的顺式中没有观察到的酶结构域-酰基转移酶对应物。在很大程度上未开发的生物合成机制中,有一类称为吡喃合酶的酶可催化由多种聚酮化合物链形成五元和六元环状醚的形成。从sorangicin装配线的第九个模块中切除的吡喃合酶结构域的1.55Å分辨率晶体结构突显了该酶与普遍存在的脱水酶结构域的相似性,并提供了对环形成机理的了解。点突变体的功能测定揭示了与脱水酶共有的活性位点组氨酸的中心重要性,以及邻近的半保守天冬酰胺的支持作用。
更新日期:2018-02-26
中文翻译:

反式酰基转移酶组装线的吡喃合酶结构域的结构和功能研究
反式-酰基转移酶装配线具有通常在其更好表征的顺式中没有观察到的酶结构域-酰基转移酶对应物。在很大程度上未开发的生物合成机制中,有一类称为吡喃合酶的酶可催化由多种聚酮化合物链形成五元和六元环状醚的形成。从sorangicin装配线的第九个模块中切除的吡喃合酶结构域的1.55Å分辨率晶体结构突显了该酶与普遍存在的脱水酶结构域的相似性,并提供了对环形成机理的了解。点突变体的功能测定揭示了与脱水酶共有的活性位点组氨酸的中心重要性,以及邻近的半保守天冬酰胺的支持作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号