当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reversibly Switchable, pH-Dependent Peptide Ligand Binding via 3,5-Diiodotyrosine Substitutions
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acschembio.8b00171 Chayanon Ngambenjawong 1 , Meilyn Sylvestre 1 , Heather H Gustafson 1 , Julio Marco B Pineda 1 , Suzie H Pun 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acschembio.8b00171 Chayanon Ngambenjawong 1 , Meilyn Sylvestre 1 , Heather H Gustafson 1 , Julio Marco B Pineda 1 , Suzie H Pun 1
Affiliation
|
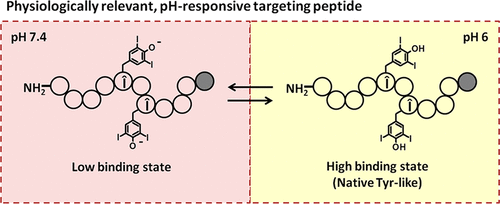
Cell type-specific targeting ligands utilized in drug delivery applications typically recognize receptors that are overexpressed on the cells of interest. Nonetheless, these receptors may also be expressed, to varying extents, on off-target cells, contributing to unintended side effects. For the selectivity profile of targeting ligands in cancer therapy to be improved, stimuli-responsive masking of these ligands with acid-, redox-, or enzyme-cleavable molecules has been reported, whereby the targeting ligands are exposed in specific environments, e.g., acidic tumor hypoxia. One possible drawback of these systems lies in their one-time, permanent trigger, which enables the “demasked” ligands to bind off-target cells if released back into the systemic circulation. A promising strategy to address the aforementioned problem is to design ligands that show selective binding based on ionization state, which may be microenvironment-dependent. In this study, we report a systematic strategy to engineer low pH-selective targeting peptides using an M2 macrophage-targeting peptide (M2pep) as an example. 3,5-Diiodotyrosine mutagenesis into native tyrosine residues of M2pep confers pH-dependent binding behavior specific to acidic environment (pH 6) when the amino acid is protonated into the native tyrosine-like state. At physiological pH of 7.4, the hydroxyl group of 3,5-diiodotyrosine on the peptide is deprotonated leading to interruption of the peptide native binding property. Our engineered pH-responsive M2pep (Ac-Y-Î-Î) binds target M2 macrophages more selectively at pH 6 than at pH 7.4. In addition, 3,5-diiodotyrosine substitutions also improve serum stability of the peptide. Finally, we demonstrate pH-dependent reversibility in target binding via a postbinding peptide elution study. The strategy presented here should be applicable for engineering pH-dependent functionality of other targeting peptides with potential applications in physiology-dependent in vivo targeting applications (e.g., targeting hypoxic tumor/inflammation) or in in vitro receptor identification.
中文翻译:

通过 3,5-二碘酪氨酸取代实现可逆转换、pH 依赖性肽配体结合
在药物递送应用中使用的细胞类型特异性靶向配体通常识别在感兴趣的细胞上过表达的受体。尽管如此,这些受体也可能在脱靶细胞上不同程度地表达,从而导致意想不到的副作用。为了改善癌症治疗中靶向配体的选择性特征,已经报道了用酸、氧化还原或酶可切割分子对这些配体的刺激响应掩蔽,由此靶向配体暴露在特定环境中,例如酸性环境肿瘤缺氧。这些系统的一个可能的缺点在于它们的一次性永久触发,如果释放回体循环,这使得“去掩蔽”的配体能够结合脱靶细胞。解决上述问题的一个有前途的策略是设计基于电离状态显示选择性结合的配体,这可能是微环境依赖性的。在本研究中,我们以 M2 巨噬细胞靶向肽 (M2pep) 为例,报告了一种设计低 pH 选择性靶向肽的系统策略。当氨基酸质子化为天然酪氨酸样状态时,3,5-二碘酪氨酸诱变到 M2pep 的天然酪氨酸残基中,赋予了酸性环境 (pH 6) 特有的 pH 依赖性结合行为。在 7.4 的生理 pH 值下,肽上 3,5-二碘酪氨酸的羟基被去质子化,导致肽天然结合特性的中断。我们设计的 pH 响应 M2pep (Ac-Y-α-β) 在 pH 6 比在 pH 7.4 时更有选择性地结合目标 M2 巨噬细胞。此外,3,5-二碘酪氨酸取代也改善了肽的血清稳定性。最后,我们通过结合后肽洗脱研究证明了靶结合的 pH 依赖性可逆性。这里介绍的策略应该适用于工程其他靶向肽的 pH 依赖性功能,在生理依赖性方面具有潜在应用体内靶向应用(例如,靶向低氧肿瘤/炎症)或体外受体鉴定。
更新日期:2018-02-26
中文翻译:

通过 3,5-二碘酪氨酸取代实现可逆转换、pH 依赖性肽配体结合
在药物递送应用中使用的细胞类型特异性靶向配体通常识别在感兴趣的细胞上过表达的受体。尽管如此,这些受体也可能在脱靶细胞上不同程度地表达,从而导致意想不到的副作用。为了改善癌症治疗中靶向配体的选择性特征,已经报道了用酸、氧化还原或酶可切割分子对这些配体的刺激响应掩蔽,由此靶向配体暴露在特定环境中,例如酸性环境肿瘤缺氧。这些系统的一个可能的缺点在于它们的一次性永久触发,如果释放回体循环,这使得“去掩蔽”的配体能够结合脱靶细胞。解决上述问题的一个有前途的策略是设计基于电离状态显示选择性结合的配体,这可能是微环境依赖性的。在本研究中,我们以 M2 巨噬细胞靶向肽 (M2pep) 为例,报告了一种设计低 pH 选择性靶向肽的系统策略。当氨基酸质子化为天然酪氨酸样状态时,3,5-二碘酪氨酸诱变到 M2pep 的天然酪氨酸残基中,赋予了酸性环境 (pH 6) 特有的 pH 依赖性结合行为。在 7.4 的生理 pH 值下,肽上 3,5-二碘酪氨酸的羟基被去质子化,导致肽天然结合特性的中断。我们设计的 pH 响应 M2pep (Ac-Y-α-β) 在 pH 6 比在 pH 7.4 时更有选择性地结合目标 M2 巨噬细胞。此外,3,5-二碘酪氨酸取代也改善了肽的血清稳定性。最后,我们通过结合后肽洗脱研究证明了靶结合的 pH 依赖性可逆性。这里介绍的策略应该适用于工程其他靶向肽的 pH 依赖性功能,在生理依赖性方面具有潜在应用体内靶向应用(例如,靶向低氧肿瘤/炎症)或体外受体鉴定。



























 京公网安备 11010802027423号
京公网安备 11010802027423号