当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Trimethylamine-N-oxide (TMAO) on Hydrophobic and Charged Interactions
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b11847 Zhaoqian Su 1 , Gopal Ravindhran 1 , Cristiano L. Dias 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b11847 Zhaoqian Su 1 , Gopal Ravindhran 1 , Cristiano L. Dias 1
Affiliation
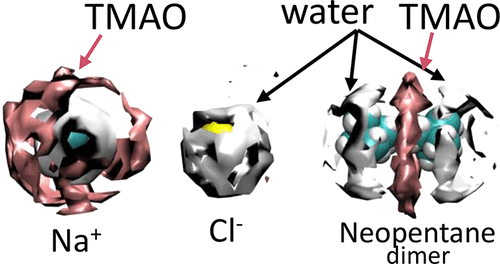
|
Effects of trimethylamine-N-oxide (TMAO) on hydrophobic and charge–charge interactions are investigated using molecular dynamics simulations. Recently, these interactions in model peptides and in the Trp-Cage miniprotein have been reported to be strongly affected by TMAO. Neopentane dimers and Na+Cl– are used, here, as models for hydrophobic and charge–charge interactions, respectively. Distance-dependent interactions, i.e., potential of mean force, are computed using an umbrella sampling protocol at different temperatures which allows us to determine enthalpy and entropic energies. We find that the large favorable entropic energy and the unfavorable enthalpy, which are characteristic of hydrophobic interactions, become smaller when TMAO is added to water. These changes account for a negligible effect and a stabilizing effect on the strength of hydrophobic interactions for simulations performed with Kast and Netz models of TMAO, respectively. Effects of TMAO on the enthalpy are mainly due to changes in terms of the potential energy involving solvent–solvent molecules. At the molecular level, TMAO is incorporated in the solvation shell of neopentane which may explain its effect on the enthalpy and entropic energy. Charge–charge interactions become stronger when TMAO is added to water because this osmolyte decreases the enthalpic penalty of bringing Na+ and Cl– close together mainly by affecting ion–solvent interactions. TMAO is attracted to Na+, becoming part of its solvation shell, whereas it is excluded from the vicinity of Cl–. These results are more pronounced for simulation performed with the Netz model which is more hydrophobic and has a larger dipole moment compared to the Kast model of TMAO.
中文翻译:

三甲胺-N-氧化物(TMAO)对疏水和带电相互作用的影响
使用分子动力学模拟研究了三甲胺-N-氧化物(TMAO)对疏水和电荷-电荷相互作用的影响。最近,据报道,TMAO强烈影响了模型肽和Trp-Cage小蛋白中的这些相互作用。新戊烷二聚体和Na + Cl –分别用作疏水和电荷-电荷相互作用的模型。距离相关的相互作用,即平均力的潜力,是使用伞形采样协议在不同温度下计算得出的,这使我们能够确定焓和熵能。我们发现,当将TMAO添加到水中时,疏水相互作用的特征,即有利的大熵能量和不利的焓变小。这些变化分别说明了用TMAO的Kast和Netz模型进行的模拟对疏水相互作用强度的影响可忽略不计和稳定化。TMAO对焓的影响主要是由于涉及溶剂-溶剂分子的势能的变化。在分子水平上 TMAO结合在新戊烷的溶剂化壳中,这可以解释其对焓和熵能的影响。当将TMAO加入水中时,电荷与电荷之间的相互作用会增强,因为这种渗透压降低了Na带来的焓变。+和Cl –主要通过影响离子与溶剂的相互作用而紧密结合。TMAO被吸引到的Na +,成为其溶剂化壳的一部分,而它选自Cl附近排除- 。与使用TMAO的Kast模型相比,Netz模型具有更大的疏水性和更大的偶极矩,对于使用Netz模型进行的仿真而言,这些结果更为明显。
更新日期:2018-02-26
中文翻译:

三甲胺-N-氧化物(TMAO)对疏水和带电相互作用的影响
使用分子动力学模拟研究了三甲胺-N-氧化物(TMAO)对疏水和电荷-电荷相互作用的影响。最近,据报道,TMAO强烈影响了模型肽和Trp-Cage小蛋白中的这些相互作用。新戊烷二聚体和Na + Cl –分别用作疏水和电荷-电荷相互作用的模型。距离相关的相互作用,即平均力的潜力,是使用伞形采样协议在不同温度下计算得出的,这使我们能够确定焓和熵能。我们发现,当将TMAO添加到水中时,疏水相互作用的特征,即有利的大熵能量和不利的焓变小。这些变化分别说明了用TMAO的Kast和Netz模型进行的模拟对疏水相互作用强度的影响可忽略不计和稳定化。TMAO对焓的影响主要是由于涉及溶剂-溶剂分子的势能的变化。在分子水平上 TMAO结合在新戊烷的溶剂化壳中,这可以解释其对焓和熵能的影响。当将TMAO加入水中时,电荷与电荷之间的相互作用会增强,因为这种渗透压降低了Na带来的焓变。+和Cl –主要通过影响离子与溶剂的相互作用而紧密结合。TMAO被吸引到的Na +,成为其溶剂化壳的一部分,而它选自Cl附近排除- 。与使用TMAO的Kast模型相比,Netz模型具有更大的疏水性和更大的偶极矩,对于使用Netz模型进行的仿真而言,这些结果更为明显。











































 京公网安备 11010802027423号
京公网安备 11010802027423号