当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Counterions on the Hydration Structure of Lanthanide Ions in Dilute Aqueous Solutions
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b12571 Valentina Migliorati 1 , Alessandra Serva 1 , Francesco Sessa 1 , Andrea Lapi 1, 2 , Paola D’Angelo 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b12571 Valentina Migliorati 1 , Alessandra Serva 1 , Francesco Sessa 1 , Andrea Lapi 1, 2 , Paola D’Angelo 1
Affiliation
|
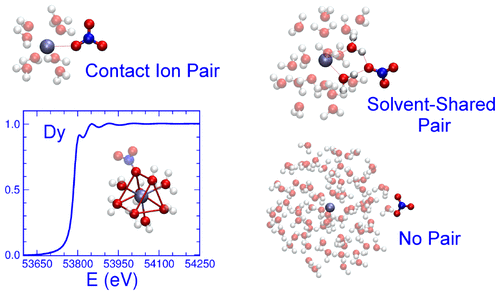
A synergic approach combining molecular dynamics (MD) simulations and X-ray absorption spectroscopy (XAS) has been used to investigate diluted (0.1 M) aqueous solutions of two lanthanide ions (Ln3+), namely, La3+ and Dy3+, with triflate, nitrate, and bis(trifluoromethylsulfonyl)imide (Tf2N–) as counterions. The different complexing ability of the three anions has been highlighted by the analysis of the MD simulations: Tf2N– does not form inner-sphere complexes, while a small amount of triflate coordinates both the La3+ and Dy3+ cations in their first solvation shell. On the other hand, the nitrate ion is almost absent in the La3+ first coordination sphere, while forming contact ion pairs with Dy3+. Both lanthanide ions are found to preferentially interact with the water molecules, and the total number of oxygen atoms coordinating the Ln3+ cations in their first solvation sphere is the same in all of the solutions, regardless of whether they belong to water molecules or to the counterion. The presence of counterions in the cation first or second shell changes neither the first shell distance nor the symmetry of the hydration complex formed in solution. The MD results have been confirmed by comparison with the Ln K-edge XAS experimental data, and the quantitative analysis of the extended X-ray absorption fine structure (EXAFS) spectra of the three salt solutions has provided a definite proof of the accuracy of the force field employed in the simulations and of the MD structural result. The anion–water and water–water hydrogen bond lifetimes have been analyzed highlighting the slow down effect of the triflate, nitrate, and Tf2N– anions on the hydrogen bond dynamics in the Ln3+ first solvation shell, with the effect being stronger in the Dy3+ solutions, due to the higher charge density of the Dy3+ ion as compared to La3+.
中文翻译:

抗衡离子对稀水溶液中镧系离子水化结构的影响
结合分子动力学(MD)模拟和X射线吸收光谱(XAS)的协同方法已用于研究两种镧系离子(Ln 3+)La 3+和Dy 3+的稀释液(0.1 M),以三氟甲磺酸盐,硝酸盐和双(三氟甲基磺酰基)酰亚胺(Tf 2 N –)作为抗衡离子。通过MD模拟分析,已突出了三种阴离子的不同络合能力:Tf 2 N –不会形成内球络合物,而少量的三氟甲磺酸盐可同时协调La 3+和Dy 3+的存在。第一个溶剂化壳中的阳离子。另一方面,在La 3+第一配位球中几乎不存在硝酸根离子,而与Dy 3+形成接触离子对。发现两种镧系元素离子优先与水分子相互作用,并且配位Ln 3+的氧原子总数在它们的第一个溶剂化领域中的阳离子在所有溶液中都是相同的,无论它们是属于水分子还是属于抗衡离子。阳离子第一或第二壳层中抗衡离子的存在既不会改变第一壳层距离,也不会改变溶液中形成的水合配合物的对称性。MD结果已通过与Ln K-edge XAS实验数据进行比较得到了证实,并且对三种盐溶液的扩展X射线吸收精细结构(EXAFS)光谱进行的定量分析为该方法的准确性提供了确定的证据。模拟和MD结构结果中使用的力场。分析了阴离子-水和水-水氢键的寿命,突出了三氟甲磺酸盐,硝酸盐和Tf 2 N –的减慢作用。阴离子对Ln 3+第一溶剂化壳中氢键动力学的影响,由于Dy 3+离子的电荷密度比La 3+高,因此在Dy 3+溶液中的作用更强。
更新日期:2018-02-26
中文翻译:

抗衡离子对稀水溶液中镧系离子水化结构的影响
结合分子动力学(MD)模拟和X射线吸收光谱(XAS)的协同方法已用于研究两种镧系离子(Ln 3+)La 3+和Dy 3+的稀释液(0.1 M),以三氟甲磺酸盐,硝酸盐和双(三氟甲基磺酰基)酰亚胺(Tf 2 N –)作为抗衡离子。通过MD模拟分析,已突出了三种阴离子的不同络合能力:Tf 2 N –不会形成内球络合物,而少量的三氟甲磺酸盐可同时协调La 3+和Dy 3+的存在。第一个溶剂化壳中的阳离子。另一方面,在La 3+第一配位球中几乎不存在硝酸根离子,而与Dy 3+形成接触离子对。发现两种镧系元素离子优先与水分子相互作用,并且配位Ln 3+的氧原子总数在它们的第一个溶剂化领域中的阳离子在所有溶液中都是相同的,无论它们是属于水分子还是属于抗衡离子。阳离子第一或第二壳层中抗衡离子的存在既不会改变第一壳层距离,也不会改变溶液中形成的水合配合物的对称性。MD结果已通过与Ln K-edge XAS实验数据进行比较得到了证实,并且对三种盐溶液的扩展X射线吸收精细结构(EXAFS)光谱进行的定量分析为该方法的准确性提供了确定的证据。模拟和MD结构结果中使用的力场。分析了阴离子-水和水-水氢键的寿命,突出了三氟甲磺酸盐,硝酸盐和Tf 2 N –的减慢作用。阴离子对Ln 3+第一溶剂化壳中氢键动力学的影响,由于Dy 3+离子的电荷密度比La 3+高,因此在Dy 3+溶液中的作用更强。











































 京公网安备 11010802027423号
京公网安备 11010802027423号