当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Parametric models to compute tryptophan fluorescence wavelengths from classical protein simulations
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-02-26 , DOI: 10.1002/jcc.25188 Alvaro J. Lopez 1 , Leandro Martínez 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-02-26 , DOI: 10.1002/jcc.25188 Alvaro J. Lopez 1 , Leandro Martínez 1
Affiliation
|
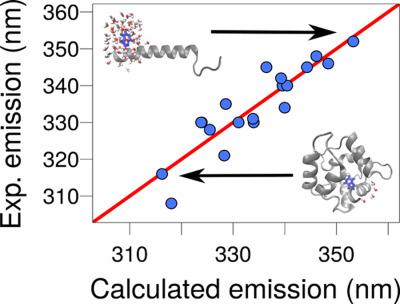
Fluorescence spectroscopy is an important method to study protein conformational dynamics and solvation structures. Tryptophan (Trp) residues are the most important and practical intrinsic probes for protein fluorescence due to the variability of their fluorescence wavelengths: Trp residues emit in wavelengths ranging from 308 to 360 nm depending on the local molecular environment. Fluorescence involves electronic transitions, thus its computational modeling is a challenging task. We show that it is possible to predict the wavelength of emission of a Trp residue from classical molecular dynamics simulations by computing the solvent‐accessible surface area or the electrostatic interaction between the indole group and the rest of the system. Linear parametric models are obtained to predict the maximum emission wavelengths with standard errors of the order 5 nm. In a set of 19 proteins with emission wavelengths ranging from 308 to 352 nm, the best model predicts the maximum wavelength of emission with a standard error of 4.89 nm and a quadratic Pearson correlation coefficient of 0.81. These models can be used for the interpretation of fluorescence spectra of proteins with multiple Trp residues, or for which local Trp environmental variability exists and can be probed by classical molecular dynamics simulations. © 2018 Wiley Periodicals, Inc.
中文翻译:

从经典蛋白质模拟计算色氨酸荧光波长的参数模型
荧光光谱是研究蛋白质构象动力学和溶剂化结构的重要方法。由于其荧光波长的可变性,色氨酸 (Trp) 残基是蛋白质荧光最重要和最实用的内在探针:Trp 残基发射的波长范围为 308 到 360 nm,具体取决于局部分子环境。荧光涉及电子跃迁,因此其计算建模是一项具有挑战性的任务。我们表明,通过计算溶剂可及的表面积或吲哚基团与系统其余部分之间的静电相互作用,可以从经典分子动力学模拟中预测 Trp 残基的发射波长。获得线性参数模型以预测最大发射波长,标准误差为 5 nm。在发射波长范围为 308 至 352 nm 的 19 种蛋白质的集合中,最佳模型预测最大发射波长的标准误差为 4.89 nm,二次 Pearson 相关系数为 0.81。这些模型可用于解释具有多个 Trp 残基的蛋白质的荧光光谱,或者存在局部 Trp 环境可变性并且可以通过经典分子动力学模拟进行探测。© 2018 Wiley Periodicals, Inc. 这些模型可用于解释具有多个 Trp 残基的蛋白质的荧光光谱,或者存在局部 Trp 环境可变性并且可以通过经典分子动力学模拟进行探测。© 2018 Wiley Periodicals, Inc. 这些模型可用于解释具有多个 Trp 残基的蛋白质的荧光光谱,或者存在局部 Trp 环境可变性并且可以通过经典分子动力学模拟进行探测。© 2018 Wiley Periodicals, Inc.
更新日期:2018-02-26
中文翻译:

从经典蛋白质模拟计算色氨酸荧光波长的参数模型
荧光光谱是研究蛋白质构象动力学和溶剂化结构的重要方法。由于其荧光波长的可变性,色氨酸 (Trp) 残基是蛋白质荧光最重要和最实用的内在探针:Trp 残基发射的波长范围为 308 到 360 nm,具体取决于局部分子环境。荧光涉及电子跃迁,因此其计算建模是一项具有挑战性的任务。我们表明,通过计算溶剂可及的表面积或吲哚基团与系统其余部分之间的静电相互作用,可以从经典分子动力学模拟中预测 Trp 残基的发射波长。获得线性参数模型以预测最大发射波长,标准误差为 5 nm。在发射波长范围为 308 至 352 nm 的 19 种蛋白质的集合中,最佳模型预测最大发射波长的标准误差为 4.89 nm,二次 Pearson 相关系数为 0.81。这些模型可用于解释具有多个 Trp 残基的蛋白质的荧光光谱,或者存在局部 Trp 环境可变性并且可以通过经典分子动力学模拟进行探测。© 2018 Wiley Periodicals, Inc. 这些模型可用于解释具有多个 Trp 残基的蛋白质的荧光光谱,或者存在局部 Trp 环境可变性并且可以通过经典分子动力学模拟进行探测。© 2018 Wiley Periodicals, Inc. 这些模型可用于解释具有多个 Trp 残基的蛋白质的荧光光谱,或者存在局部 Trp 环境可变性并且可以通过经典分子动力学模拟进行探测。© 2018 Wiley Periodicals, Inc.











































 京公网安备 11010802027423号
京公网安备 11010802027423号