当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cooperativity between hydrogen- and halogen bonds: the case of selenourea†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c8cp00353j Gianluca Ciancaleoni 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c8cp00353j Gianluca Ciancaleoni 1, 2, 3, 4
Affiliation
|
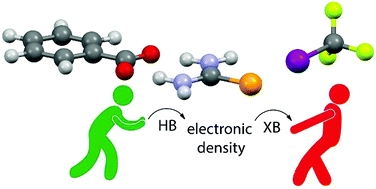
A combined experimental/computational study of cooperativity between halogen-(XB) and hydrogen bonding (HB) is presented. Selenourea (SeU) has been chosen due to its ability to act at the same time as an XB acceptor toward I(CF2)5CF3 (I1) through the two lone pairs on the selenium atom, and as a HB donor to the benzoate anion through its two amino moieties. All equilibrium constants have been estimated using either diffusion NMR and NMR titration techniques. Experimental results demonstrate that the –NH2⋯anion interaction strongly enhances the Se⋯I one of about one order of magnitude (in terms of formation constant of the adduct), whereas DFT results rationalize such results revealing that the presence of a HB between the benzoate and SeU strongly polarizes the latter, enhancing the negative partial charge on selenium and, consequently, its Lewis basicity and its XB acceptor properties.
中文翻译:

氢键和卤素键之间的协同作用:硒硒†
提出了结合实验/计算研究的卤素-(XB)和氢键(HB)之间的协同作用。之所以选择Selenourea(SeU),是因为它具有与XB受体同时通过硒原子上的两个孤对作用于I(CF 2)5 CF 3(I1)的能力,以及作为XB受体的HB供体。通过其两个氨基部分形成苯甲酸酯阴离子。使用扩散NMR和NMR滴定技术都可以估算所有平衡常数。实验结果表明,–NH 2阴离子相互作用强烈地增强了Se⋯I约一个数量级(就加合物的形成常数而言),而DFT结果使这些结果合理化,表明苯甲酸盐和SeU之间存在HB强烈极化了后者。增强硒的负部分电荷,从而增强其Lewis碱性和XB受体性能。
更新日期:2018-02-26
中文翻译:

氢键和卤素键之间的协同作用:硒硒†
提出了结合实验/计算研究的卤素-(XB)和氢键(HB)之间的协同作用。之所以选择Selenourea(SeU),是因为它具有与XB受体同时通过硒原子上的两个孤对作用于I(CF 2)5 CF 3(I1)的能力,以及作为XB受体的HB供体。通过其两个氨基部分形成苯甲酸酯阴离子。使用扩散NMR和NMR滴定技术都可以估算所有平衡常数。实验结果表明,–NH 2阴离子相互作用强烈地增强了Se⋯I约一个数量级(就加合物的形成常数而言),而DFT结果使这些结果合理化,表明苯甲酸盐和SeU之间存在HB强烈极化了后者。增强硒的负部分电荷,从而增强其Lewis碱性和XB受体性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号