当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituent influence in phenanthroline-derived ancillary ligands on the excited state nature of novel cationic Ir(iii) complexes†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c8nj00334c Iván González 1, 2, 3, 4, 5 , Mirco Natali 6, 7, 8, 9, 10 , Alan R. Cabrera 4, 5, 11, 12, 13 , Bárbara Loeb 4, 5, 11, 12, 13 , Jerónimo Maze 4, 5, 14 , Paulina Dreyse 5, 15, 16
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c8nj00334c Iván González 1, 2, 3, 4, 5 , Mirco Natali 6, 7, 8, 9, 10 , Alan R. Cabrera 4, 5, 11, 12, 13 , Bárbara Loeb 4, 5, 11, 12, 13 , Jerónimo Maze 4, 5, 14 , Paulina Dreyse 5, 15, 16
Affiliation
|
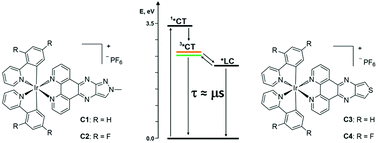
In the quest for coordination compounds with potential applications in energy conversion processes, a new series of four Ir(III) complexes (C1–4) of the type [Ir(R-ppy)2(Ln)](PF6), where R-ppy = 2-phenylpyridine (ppy) or 2,4-difluorophenylpyridine (F2-ppy) and Ln = 1-methyl-1H-pyrazole[3′,4′:5,6]pyrazino[2,3-f][1,10]phenanthroline (L1) or thieno[3′,4′:5,6]pyrazino[2,3-f][1,10]phenanthroline (L2) has been synthesized. The photophysical properties of these compounds have been thoroughly characterized by both steady-state and time-resolved spectroscopic techniques, pointing out a complex interplay between excited states of different nature that plays a crucial role in the deactivation processes. In the case of complexes C1–2 that feature the same L1 ancillary ligand, the lowest excited states at room temperature are characterized by an admixture between the 3MLCT/3LLCT and 3LC states, with an almost pure 3LC character in C2. For C3–4, the admixture among charge-transfer and ligand-centred states is negligible, due to the appreciably low energy of the LC one, which, however, plays a non-innocent role in the deactivation pathway of the triplet charge-transfer emissive states of complexes C3–4. This work thus highlights the importance of a detailed comprehension of the photophysical properties of Ir(III) complexes in view of their use in energy transformation systems.
中文翻译:

菲咯啉衍生的辅助配体中取代基对新型阳离子Ir(iii)配合物的激发态性质的影响†
为了寻求在能量转换过程中具有潜在应用价值的配位化合物,新的一系列四个[Ir(R-ppy)2(Ln)](PF 6)类型的Ir(III)配合物(C1-4),其中R-ppy = 2-苯基吡啶(ppy)或2,4-二氟苯基吡啶(F 2 -ppy),Ln = 1-甲基-1 H-吡唑[3',4':5,6]吡嗪并[2,3- f ] [1,10]菲咯啉(L1)或噻吩并[3',4':5,6] pyrazino [2,3- f ] [1,10]菲咯啉(L2)已合成。这些化合物的光物理性质已通过稳态和时间分辨光谱技术进行了全面表征,指出了不同性质的激发态之间的复杂相互作用,在失活过程中起着至关重要的作用。对于具有相同L1辅助配体的复合物C1-2,在室温下最低的激发态的特征是3 MLCT / 3 LLCT和3 LC态之间的混合物,而C2中几乎具有纯3 LC特性。对于C3–4,由于LC一的能量非常低,因此电荷转移状态和配体中心状态之间的混合可以忽略不计,但是,它在配合物三重态电荷转移发射态的失活途径中起着无害的作用C3–4。因此,鉴于其在能量转换系统中的使用,这项工作凸显了对Ir(III)配合物的光物理性质进行详细理解的重要性。
更新日期:2018-02-26
中文翻译:

菲咯啉衍生的辅助配体中取代基对新型阳离子Ir(iii)配合物的激发态性质的影响†
为了寻求在能量转换过程中具有潜在应用价值的配位化合物,新的一系列四个[Ir(R-ppy)2(Ln)](PF 6)类型的Ir(III)配合物(C1-4),其中R-ppy = 2-苯基吡啶(ppy)或2,4-二氟苯基吡啶(F 2 -ppy),Ln = 1-甲基-1 H-吡唑[3',4':5,6]吡嗪并[2,3- f ] [1,10]菲咯啉(L1)或噻吩并[3',4':5,6] pyrazino [2,3- f ] [1,10]菲咯啉(L2)已合成。这些化合物的光物理性质已通过稳态和时间分辨光谱技术进行了全面表征,指出了不同性质的激发态之间的复杂相互作用,在失活过程中起着至关重要的作用。对于具有相同L1辅助配体的复合物C1-2,在室温下最低的激发态的特征是3 MLCT / 3 LLCT和3 LC态之间的混合物,而C2中几乎具有纯3 LC特性。对于C3–4,由于LC一的能量非常低,因此电荷转移状态和配体中心状态之间的混合可以忽略不计,但是,它在配合物三重态电荷转移发射态的失活途径中起着无害的作用C3–4。因此,鉴于其在能量转换系统中的使用,这项工作凸显了对Ir(III)配合物的光物理性质进行详细理解的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号