当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Binding of Barbital to a Synthetic Macrocyclic Receptor. A Charge Density Study
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.7b11674 Jonathan J. Du 1 , Jane R. Hanrahan 1 , V. Raja Solomon 1 , Peter A. Williams 1, 2 , Paul W. Groundwater 1 , Jacob Overgaard 3 , James A. Platts 4 , David E. Hibbs 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.7b11674 Jonathan J. Du 1 , Jane R. Hanrahan 1 , V. Raja Solomon 1 , Peter A. Williams 1, 2 , Paul W. Groundwater 1 , Jacob Overgaard 3 , James A. Platts 4 , David E. Hibbs 1
Affiliation
|
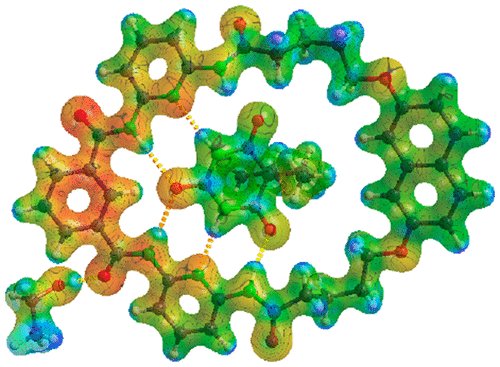
Experimental charge density distribution studies, complemented by quantum mechanical theoretical calculations, of a host–guest system composed of a macrocycle (1) and barbital (2) in a 1:1 ratio (3) have been carried out via high-resolution single-crystal X-ray diffraction. The data were modeled using the conventional multipole model of electron density according to the Hansen–Coppens formalism. The asymmetric unit of macrocycle 1 contained an intraannular ethanol molecule and an extraannular acetonitrile molecule, and the asymmetric unit of 3 also contained an intraannular ethanol molecule. Visual comparison of the conformations of the macrocyclic ring shows the rotation by 180° of an amide bond attributed to competitive hydrogen bonding. It was found that the intraannular and extraannular molecules inside were orientated to maximize the number of hydrogen bonds present, with the presence of barbital in 3 resulting in the greatest stabilization. Hydrogen bonds ranging in strength from 4 to 70 kJ mol–1 were the main stabilizing force. Further analysis of the electrostatic potential among 1, 2, and 3 showed significant charge redistribution when cocrystallization occurred, which was further confirmed by a comparison of atomic charges. The findings presented herein introduce the possibility of high-resolution X-ray crystallography playing a more prominent role in the drug design process.
中文翻译:

探索巴比妥与合成的大环受体的结合。电荷密度研究
通过高分辨率的单分子束,对由大环(1)和巴比妥(2)以1:1的比例(3)组成的主客体系统进行了实验电荷密度分布研究,并辅以量子力学理论计算。晶体X射线衍射。根据Hansen-Coppens形式主义,使用常规的电子密度多极模型对数据进行建模。大环1的不对称单元包含一个环内乙醇分子和一个环外乙腈分子,而不对称单元3还包含一个环内乙醇分子。大环环构象的视觉比较显示归因于竞争性氢键的酰胺键旋转180°。发现内部的环内和环外分子被定向以最大化存在的氢键的数目,其中巴比妥的存在3导致最大的稳定性。主要的稳定力是氢键,强度范围为4至70 kJ mol –1。之间的静电势的进一步分析1,2,和3当发生共结晶时,H2O3会显示出明显的电荷再分布,这通过原子电荷的比较进一步得到证实。本文介绍的发现介绍了高分辨率X射线晶体学在药物设计过程中发挥更重要作用的可能性。
更新日期:2018-02-26
中文翻译:

探索巴比妥与合成的大环受体的结合。电荷密度研究
通过高分辨率的单分子束,对由大环(1)和巴比妥(2)以1:1的比例(3)组成的主客体系统进行了实验电荷密度分布研究,并辅以量子力学理论计算。晶体X射线衍射。根据Hansen-Coppens形式主义,使用常规的电子密度多极模型对数据进行建模。大环1的不对称单元包含一个环内乙醇分子和一个环外乙腈分子,而不对称单元3还包含一个环内乙醇分子。大环环构象的视觉比较显示归因于竞争性氢键的酰胺键旋转180°。发现内部的环内和环外分子被定向以最大化存在的氢键的数目,其中巴比妥的存在3导致最大的稳定性。主要的稳定力是氢键,强度范围为4至70 kJ mol –1。之间的静电势的进一步分析1,2,和3当发生共结晶时,H2O3会显示出明显的电荷再分布,这通过原子电荷的比较进一步得到证实。本文介绍的发现介绍了高分辨率X射线晶体学在药物设计过程中发挥更重要作用的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号