当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics Study of Heterogeneous Bromine Release from the Reaction between Gaseous Ozone and Aqueous Bromide Solution
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.7b12819 Yosuke Sakamoto , Motoki Goda , Jun Hirokawa
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.7b12819 Yosuke Sakamoto , Motoki Goda , Jun Hirokawa
|
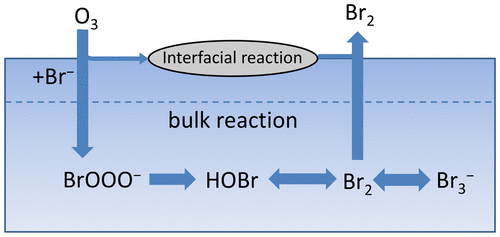
The heterogeneous release of molecular bromine, Br2, from the reaction between gaseous ozone and aqueous bromide ion in seawater ice and sea salt aerosols is considered to be an initial source of reactive bromine species in the troposphere. Recent studies have demonstrated that the uptake of ozone by aqueous bromide solution is promoted by reactions at the gas–liquid interface. The present work investigated the heterogeneous reaction between gaseous ozone and aqueous bromide solution at atmospheric pressure and room temperature using a wetted wall flow reactor combined with a chemical ionization mass spectrometer. The emission rate of Br2 was measured as a function of gaseous ozone concentration, aqueous bromide concentration, and pH. In addition, we conducted a simple kinetics model simulation that included only bulk aqueous-phase reactions and compared the theoretical values with the experimentally determined values. The Br2 emission rates measured experimentally differ from the simulated rates at relatively high bromide concentration, as well as in the pH region of 6–9. These differences might be explained by different Br– concentration and/or deprotonation efficiency near the interface region and those in the bulk solution.
中文翻译:

气态臭氧与溴化水溶液反应释放异质溴的动力学研究
从气态臭氧与海水冰和海盐气溶胶中的溴化氢离子之间的气态反应,分子溴Br 2的异质释放被认为是对流层反应性溴物种的最初来源。最近的研究表明,气液界面的反应促进了溴化物水溶液对臭氧的吸收。本工作使用湿壁流反应器结合化学电离质谱仪研究了大气压下和室温下气态臭氧与溴化水溶液之间的异相反应。Br 2的发射率所测得的H 2 O 3是气态臭氧浓度,溴化水溶液浓度和pH的函数。此外,我们进行了简单的动力学模型模拟,仅包括本体水相反应,并将理论值与实验确定的值进行了比较。在相对较高的溴化物浓度下以及在6–9的pH范围内,实验测量的Br 2排放速率与模拟的速率不同。这些差异可能是由不同的溴进行说明-的浓度和/或附近的界面区域去质子化效率和那些在本体溶液中。
更新日期:2018-02-26
中文翻译:

气态臭氧与溴化水溶液反应释放异质溴的动力学研究
从气态臭氧与海水冰和海盐气溶胶中的溴化氢离子之间的气态反应,分子溴Br 2的异质释放被认为是对流层反应性溴物种的最初来源。最近的研究表明,气液界面的反应促进了溴化物水溶液对臭氧的吸收。本工作使用湿壁流反应器结合化学电离质谱仪研究了大气压下和室温下气态臭氧与溴化水溶液之间的异相反应。Br 2的发射率所测得的H 2 O 3是气态臭氧浓度,溴化水溶液浓度和pH的函数。此外,我们进行了简单的动力学模型模拟,仅包括本体水相反应,并将理论值与实验确定的值进行了比较。在相对较高的溴化物浓度下以及在6–9的pH范围内,实验测量的Br 2排放速率与模拟的速率不同。这些差异可能是由不同的溴进行说明-的浓度和/或附近的界面区域去质子化效率和那些在本体溶液中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号