当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Composition of Free Mixed NaCl/Na2SO4 Nanoscale Aerosols Probed by X-ray Photoelectron Spectroscopy
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.8b00615 E. Antonsson 1 , C. Raschpichler 1 , B. Langer 1 , D. Marchenko 1 , E. Rühl 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.8b00615 E. Antonsson 1 , C. Raschpichler 1 , B. Langer 1 , D. Marchenko 1 , E. Rühl 1
Affiliation
|
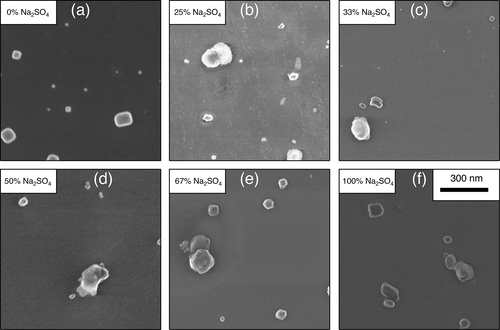
The local chemical surface composition of unsupported mixed solid NaCl/Na2SO4 aerosols (d ∼ 70 nm) is studied by X-ray photoelectron spectroscopy. The solid aerosols are generated by drying aqueous droplets containing mixtures of the two salts in different mole fractions. The mole fraction of these salts is found to deviate at the solid aerosol surface significantly from the initial droplet composition. The minority species in the droplets are found to be enhanced at the surface of the solid mixed aerosols. This surface enhancement is rationalized in terms of the nucleation/crystallization process, where the salts evidently do not cocrystallize, rather than each salt forms pure crystal moieties. Characteristic variations of the surface ion concentration as a function of the mole fraction of the salts in the initial droplet are observed in the nanometer size regime. This is unlike core–shell architectures previously found in mixed micron salt aerosols, indicating that aerosol models derived from micron-sized aerosols are evidently not fully reliable to describe the surface composition of nanosized aerosols. Furthermore, surface enhancement of the minority component in mixed NaCl/Na2SO4 aerosols is also different from previous results on surface segregation of mixed NaCl/NaBr aerosols, where one of the anionic species is surface segregated for all mole fractions, which was explained in terms of the ability of the involved salts to cocrystallize and forming solid solutions. The present results rather indicate that mixed NaCl/Na2SO4 aerosols do not cocrystallize. Electron microscopy of deposited mixed salt aerosols reveals mostly a cubic structure of pure NaCl aerosols, whereas mixed salt aerosols are found to show a grainy structure composed of multiple small crystals which supports the present findings obtained from photoelectron spectroscopy.
中文翻译:

X射线光电子能谱探测的游离混合NaCl / Na 2 SO 4纳米气溶胶的表面组成
无载体的固体NaCl / Na 2 SO 4混合气溶胶的局部化学表面组成(dX射线光电子能谱研究了约70 nm左右)。固体气雾剂是通过干燥包含两种摩尔比例不同的两种盐的混合物的水滴而产生的。发现这些盐的摩尔分数在固体气雾剂表面处明显偏离初始液滴组成。发现液滴中的少数种类在固体混合气雾剂的表面处被增强。在成核/结晶过程方面,这种表面增强是合理的,其中盐显然不共结晶,而不是每种盐形成纯的晶体部分。在纳米尺寸范围内观察到表面离子浓度随初始液滴中盐的摩尔分数而变化的特征变化。这与以前在混合微米盐气溶胶中发现的核-壳结构不同,这表明源自微米级气溶胶的气溶胶模型显然不能完全可靠地描述纳米级气溶胶的表面组成。此外,混合NaCl / Na中少数组分的表面增强2 SO 4气雾剂也不同于以前的混合NaCl / NaBr气雾剂表面偏析的结果,其中一种阴离子物质在所有摩尔分数中均处于表面偏析状态,这是根据涉及的盐共结晶和形成的能力来解释的固溶体。目前的结果表明,混合的NaCl / Na 2 SO 4气雾剂不会共结晶。沉积的混合盐气溶胶的电子显微镜显示大部分为纯NaCl气溶胶的立方结构,而混合盐气溶胶显示为由多个小晶体组成的粒状结构,这支持了从光电子能谱学获得的当前发现。
更新日期:2018-02-26
中文翻译:

X射线光电子能谱探测的游离混合NaCl / Na 2 SO 4纳米气溶胶的表面组成
无载体的固体NaCl / Na 2 SO 4混合气溶胶的局部化学表面组成(dX射线光电子能谱研究了约70 nm左右)。固体气雾剂是通过干燥包含两种摩尔比例不同的两种盐的混合物的水滴而产生的。发现这些盐的摩尔分数在固体气雾剂表面处明显偏离初始液滴组成。发现液滴中的少数种类在固体混合气雾剂的表面处被增强。在成核/结晶过程方面,这种表面增强是合理的,其中盐显然不共结晶,而不是每种盐形成纯的晶体部分。在纳米尺寸范围内观察到表面离子浓度随初始液滴中盐的摩尔分数而变化的特征变化。这与以前在混合微米盐气溶胶中发现的核-壳结构不同,这表明源自微米级气溶胶的气溶胶模型显然不能完全可靠地描述纳米级气溶胶的表面组成。此外,混合NaCl / Na中少数组分的表面增强2 SO 4气雾剂也不同于以前的混合NaCl / NaBr气雾剂表面偏析的结果,其中一种阴离子物质在所有摩尔分数中均处于表面偏析状态,这是根据涉及的盐共结晶和形成的能力来解释的固溶体。目前的结果表明,混合的NaCl / Na 2 SO 4气雾剂不会共结晶。沉积的混合盐气溶胶的电子显微镜显示大部分为纯NaCl气溶胶的立方结构,而混合盐气溶胶显示为由多个小晶体组成的粒状结构,这支持了从光电子能谱学获得的当前发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号