当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Rate Coefficients and Kinetic Isotope Effects for the Reaction OH + CH4 → H2O + CH3 on an ab Initio-Based Potential Energy Surface
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.8b01201 Jun Li 1 , Hua Guo 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acs.jpca.8b01201 Jun Li 1 , Hua Guo 2
Affiliation
|
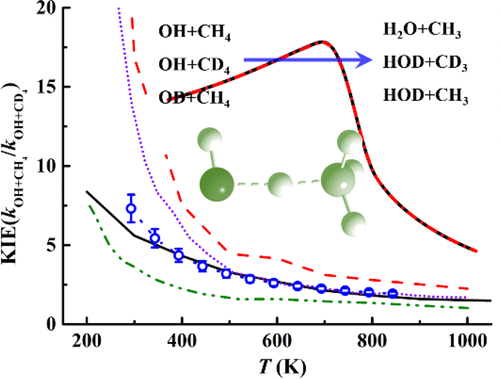
Thermal rate coefficients for the title reaction and its various isotopologues are computed using a tunneling-corrected transition-state theory on a global potential energy surface recently developed by fitting a large number of high-level ab initio points. The calculated rate coefficients are found to agree well with the measured ones in a wide temperature range, validating the accuracy of the potential energy surface. Strong non-Arrhenius effects are found at low temperatures. In addition, the calculations reproduced the primary and secondary kinetic isotope effects. These results confirm the strong influence of tunneling to this heavy-light-heavy hydrogen abstraction reaction.
中文翻译:

从头算基于势能面的OH + CH 4 →H 2 O + CH 3反应的热速率系数和动力学同位素效应
使用隧道校正的过渡态理论,通过拟合大量高级从头算点,在最近开发的全局势能表面上使用隧道校正的过渡态理论,计算了标题反应及其各种同位素的热速率系数。发现计算出的速率系数在很宽的温度范围内与测得的速率系数非常吻合,从而验证了势能面的准确性。在低温下发现很强的非阿累尼乌斯效应。此外,计算结果重现了一级和二级动力学同位素效应。这些结果证实了隧穿对该重-轻-重氢提取反应的强烈影响。
更新日期:2018-02-26
中文翻译:

从头算基于势能面的OH + CH 4 →H 2 O + CH 3反应的热速率系数和动力学同位素效应
使用隧道校正的过渡态理论,通过拟合大量高级从头算点,在最近开发的全局势能表面上使用隧道校正的过渡态理论,计算了标题反应及其各种同位素的热速率系数。发现计算出的速率系数在很宽的温度范围内与测得的速率系数非常吻合,从而验证了势能面的准确性。在低温下发现很强的非阿累尼乌斯效应。此外,计算结果重现了一级和二级动力学同位素效应。这些结果证实了隧穿对该重-轻-重氢提取反应的强烈影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号