Synthesis ( IF 2.2 ) Pub Date : 2018-02-26 , DOI: 10.1055/s-0036-1591937 Luca Beverina 1, 2 , Luca Vaghi 1 , Alessandro Sanzone 1 , Mauro Sassi 1, 2 , Simone Pagani 1 , Antonio Papagni 1
|
Abstract
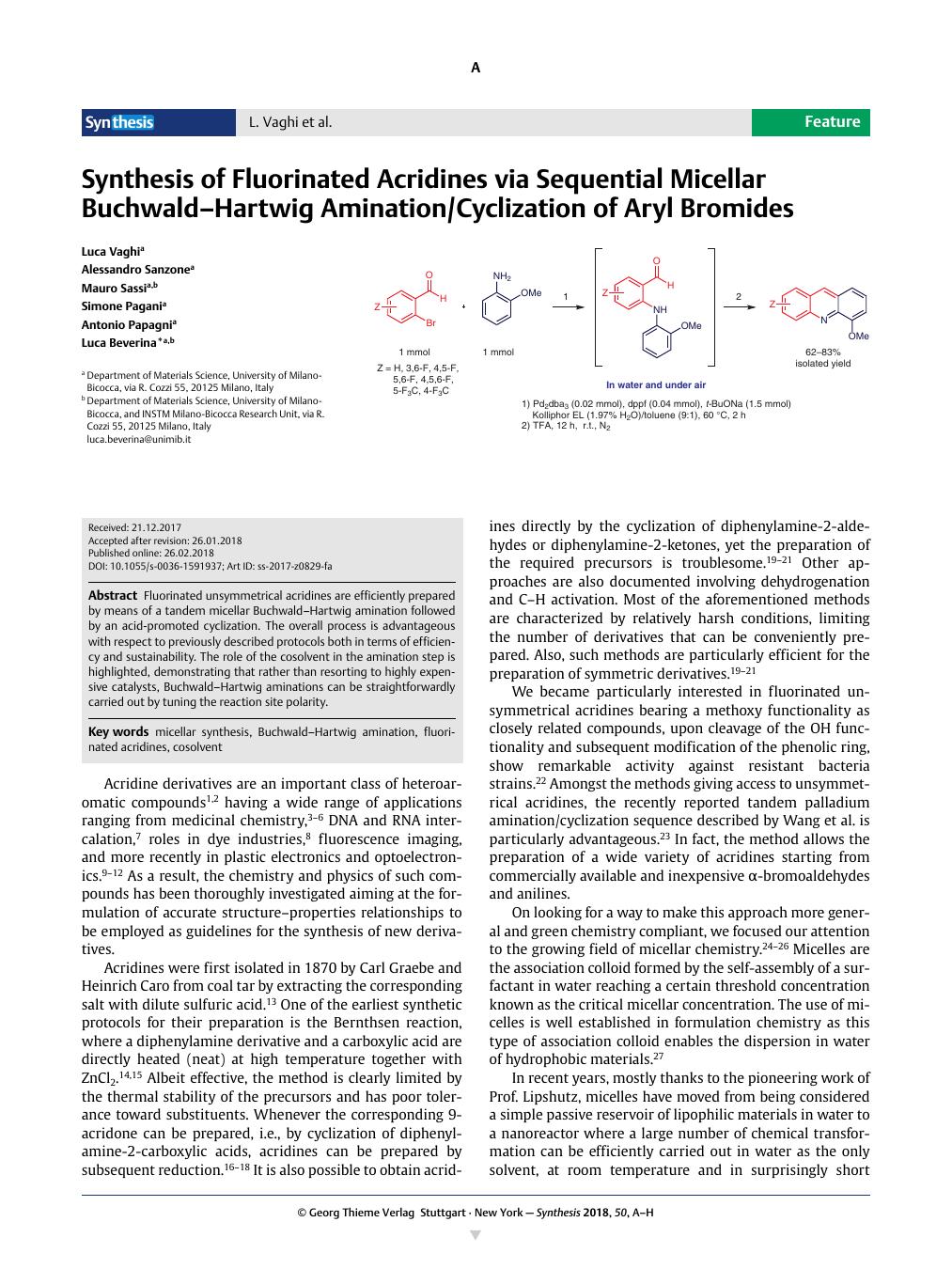
Fluorinated unsymmetrical acridines are efficiently prepared by means of a tandem micellar Buchwald–Hartwig amination followed by an acid-promoted cyclization. The overall process is advantageous with respect to previously described protocols both in terms of efficiency and sustainability. The role of the cosolvent in the amination step is highlighted, demonstrating that rather than resorting to highly expensive catalysts, Buchwald–Hartwig aminations can be straightforwardly carried out by tuning the reaction site polarity.
Fluorinated unsymmetrical acridines are efficiently prepared by means of a tandem micellar Buchwald–Hartwig amination followed by an acid-promoted cyclization. The overall process is advantageous with respect to previously described protocols both in terms of efficiency and sustainability. The role of the cosolvent in the amination step is highlighted, demonstrating that rather than resorting to highly expensive catalysts, Buchwald–Hartwig aminations can be straightforwardly carried out by tuning the reaction site polarity.
中文翻译:

通过顺序胶束布赫瓦尔德-哈特维格胺化/芳基溴化物的环化反应合成氟化A啶
摘要
氟化不对称a啶可通过串联胶束布赫瓦尔德-哈特维格胺胺化,然后进行酸促进的环化反应而有效制备。相对于先前描述的协议,整个过程在效率和可持续性方面都是有利的。强调了助溶剂在胺化步骤中的作用,这表明,通过调节反应位点的极性,可以直接进行Buchwald-Hartwig胺化,而不是使用昂贵的催化剂。
氟化不对称a啶可通过串联胶束布赫瓦尔德-哈特维格胺胺化,然后进行酸促进的环化反应而有效制备。相对于先前描述的协议,整个过程在效率和可持续性方面都是有利的。强调了助溶剂在胺化步骤中的作用,这表明,通过调节反应位点的极性,可以直接进行Buchwald-Hartwig胺化,而不是使用昂贵的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号