当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
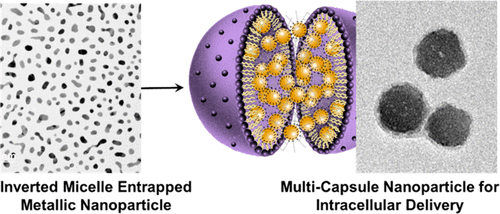
Facile Chemical Strategy to Hydrophobically Modify Solid Nanoparticles Using Inverted Micelle-Based Multicapsule for Efficient Intracellular Delivery
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00061 Enrique A. Daza 1 , Aaron S. Schwartz-Duval 1 , Kimberly Volkman , Dipanjan Pan 1
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00061 Enrique A. Daza 1 , Aaron S. Schwartz-Duval 1 , Kimberly Volkman , Dipanjan Pan 1
Affiliation
|
Theranostic nanoparticles have incredible potential for biomedical applications by enabling visual confirmation of therapeutic efficacy. Numerous issues challenge their clinical translation and are primarily related to the complex chemistry and scalability of synthesizing Nanoparticles. We report a 2-step chemical strategy for high-throughput intracellular delivery of organic and inorganic solid nanoparticles. This process takes an additional step beyond hydrophobic surface modification facilitated by inverted micelle transfer, toward the packing of multiple solid nanoparticles into a soft-shelled lipid capsule, termed the Nano-multicapsule (NMC). This technique is high yielding and does not require the complex purification steps in anaerobic/hydrophobic reactions for hydrophobic modification. To demonstrate the efficacy across different material compositions, we separately entrapped ∼10 nm gold and carbon nanoparticles (AuNP and CNP) within inverted micelles, and subsequently NMCs, then quantified their internalization in a human breast cancer cell line. For encapsulated AuNPs (NMC-AuNP), we confirmed greater cellular internalization of gold through ICP-OES and TEM analyses. Raman spectroscopic analysis of cells treated with encapsulated CNPs (NMC-CNP) also exhibited high degrees of uptake with apparent intracellular localization as opposed to free CNP treatment.
中文翻译:

利用逆胶束基多胶囊疏水修饰固体纳米粒子的高效化学策略,可实现高效的细胞内递送
通过对治疗功效的目视确认,治疗治疗性纳米颗粒在生物医学应用中具有不可思议的潜力。许多问题挑战了它们的临床翻译,并且主要与合成纳米颗粒的复杂化学和可扩展性有关。我们报告了有机和无机固体纳米粒子的高通量细胞内传递的两步化学策略。除了通过反向胶束转移促进的疏水表面改性外,该过程还需要朝着将多个固体纳米颗粒填充到被称为纳米多胶囊(NMC)的软脂质胶囊中的步骤。该技术产率高,不需要厌氧/疏水反应中复杂的纯化步骤即可进行疏水改性。为了证明在不同材料成分上的功效,我们分别将约10 nm的金和碳纳米颗粒(AuNP和CNP)包埋在倒置的胶束中,然后在NMC中包埋,然后定量其在人乳腺癌细胞系中的内在化。对于封装的AuNPs(NMC-AuNP),我们通过ICP-OES和TEM分析证实了金的更大细胞内在化。与游离的CNP处理相反,用胶囊化的CNP(NMC-CNP)处理的细胞的拉曼光谱分析也表现出高度的摄取,具有明显的细胞内定位。我们通过ICP-OES和TEM分析证实了金的更大细胞内在化。与游离的CNP处理相反,用胶囊化的CNP(NMC-CNP)处理的细胞的拉曼光谱分析也表现出高度的摄取,具有明显的细胞内定位。我们通过ICP-OES和TEM分析证实了金的更大细胞内在化。与游离的CNP处理相反,用胶囊化的CNP(NMC-CNP)处理的细胞的拉曼光谱分析也表现出高度的摄取,具有明显的细胞内定位。
更新日期:2018-02-26
中文翻译:

利用逆胶束基多胶囊疏水修饰固体纳米粒子的高效化学策略,可实现高效的细胞内递送
通过对治疗功效的目视确认,治疗治疗性纳米颗粒在生物医学应用中具有不可思议的潜力。许多问题挑战了它们的临床翻译,并且主要与合成纳米颗粒的复杂化学和可扩展性有关。我们报告了有机和无机固体纳米粒子的高通量细胞内传递的两步化学策略。除了通过反向胶束转移促进的疏水表面改性外,该过程还需要朝着将多个固体纳米颗粒填充到被称为纳米多胶囊(NMC)的软脂质胶囊中的步骤。该技术产率高,不需要厌氧/疏水反应中复杂的纯化步骤即可进行疏水改性。为了证明在不同材料成分上的功效,我们分别将约10 nm的金和碳纳米颗粒(AuNP和CNP)包埋在倒置的胶束中,然后在NMC中包埋,然后定量其在人乳腺癌细胞系中的内在化。对于封装的AuNPs(NMC-AuNP),我们通过ICP-OES和TEM分析证实了金的更大细胞内在化。与游离的CNP处理相反,用胶囊化的CNP(NMC-CNP)处理的细胞的拉曼光谱分析也表现出高度的摄取,具有明显的细胞内定位。我们通过ICP-OES和TEM分析证实了金的更大细胞内在化。与游离的CNP处理相反,用胶囊化的CNP(NMC-CNP)处理的细胞的拉曼光谱分析也表现出高度的摄取,具有明显的细胞内定位。我们通过ICP-OES和TEM分析证实了金的更大细胞内在化。与游离的CNP处理相反,用胶囊化的CNP(NMC-CNP)处理的细胞的拉曼光谱分析也表现出高度的摄取,具有明显的细胞内定位。



























 京公网安备 11010802027423号
京公网安备 11010802027423号