当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of Novel Deuterated Ligands Functionally Selective for the γ-Aminobutyric Acid Type A Receptor (GABAAR) α6 Subtype with Improved Metabolic Stability and Enhanced Bioavailability.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-03-06 , DOI: 10.1021/acs.jmedchem.7b01664 Daniel E Knutson,Revathi Kodali,Branka Divović,Marco Treven,Michael R Stephen,Nicolas M Zahn,Vladimir Dobričić,Alec T Huber,Matheus A Meirelles,Ranjit S Verma,Laurin Wimmer,Christopher Witzigmann,Leggy A Arnold,Lih-Chu Chiou,Margot Ernst,Marko D Mihovilovic,Miroslav M Savić,Werner Sieghart,James M Cook
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-03-06 , DOI: 10.1021/acs.jmedchem.7b01664 Daniel E Knutson,Revathi Kodali,Branka Divović,Marco Treven,Michael R Stephen,Nicolas M Zahn,Vladimir Dobričić,Alec T Huber,Matheus A Meirelles,Ranjit S Verma,Laurin Wimmer,Christopher Witzigmann,Leggy A Arnold,Lih-Chu Chiou,Margot Ernst,Marko D Mihovilovic,Miroslav M Savić,Werner Sieghart,James M Cook
|
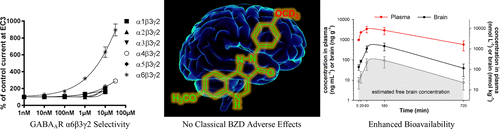
Recent reports indicate that α6β2/3γ2 GABAAR selective ligands may be important for the treatment of trigeminal activation-related pain and neuropsychiatric disorders with sensori-motor gating deficits. Based on 3 functionally α6β2/3γ2 GABAAR selective pyrazoloquinolinones, 42 novel analogs were synthesized, and their in vitro metabolic stability and cytotoxicity as well as their in vivo pharmacokinetics, basic behavioral pharmacology, and effects on locomotion were investigated. Incorporation of deuterium into the methoxy substituents of the ligands increased their duration of action via improved metabolic stability and bioavailability, while their selectivity for the GABAAR α6 subtype was retained. 8b was identified as the lead compound with a substantially improved pharmacokinetic profile. The ligands allosterically modulated diazepam insensitive α6β2/3γ2 GABAARs and were functionally silent at diazepam sensitive α1β2/3γ2 GABAARs, thus no sedation was detected. In addition, these analogs were not cytotoxic, which render them interesting candidates for treatment of CNS disorders mediated by GABAAR α6β2/3γ2 subtypes.
中文翻译:

设计和合成对 γ-氨基丁酸 A 型受体 (GABAAR) α6 亚型具有功能选择性的新型氘代配体,具有改善的代谢稳定性和增强的生物利用度。
最近的报告表明,α6β2/3γ2 GABAAR 选择性配体可能对治疗三叉神经激活相关的疼痛和感觉运动门控缺陷的神经精神疾病很重要。基于 3 种功能性 α6β2/3γ2 GABAAR 选择性吡唑喹啉酮,合成了 42 种新型类似物,并研究了它们的体外代谢稳定性和细胞毒性以及它们的体内药代动力学、基本行为药理学和对运动的影响。将氘掺入配体的甲氧基取代基通过改善代谢稳定性和生物利用度增加了它们的作用持续时间,同时保留了它们对 GABAAR α6 亚型的选择性。8b 被确定为具有显着改善的药代动力学特征的先导化合物。配体对地西泮不敏感的α6β2/3γ2 GABAARs 进行变构调节,并且对地西泮敏感的α1β2/3γ2 GABAARs 功能沉默,因此未检测到镇静作用。此外,这些类似物没有细胞毒性,这使它们成为治疗由 GABAAR α6β2/3γ2 亚型介导的 CNS 疾病的有趣候选者。
更新日期:2018-02-26
中文翻译:

设计和合成对 γ-氨基丁酸 A 型受体 (GABAAR) α6 亚型具有功能选择性的新型氘代配体,具有改善的代谢稳定性和增强的生物利用度。
最近的报告表明,α6β2/3γ2 GABAAR 选择性配体可能对治疗三叉神经激活相关的疼痛和感觉运动门控缺陷的神经精神疾病很重要。基于 3 种功能性 α6β2/3γ2 GABAAR 选择性吡唑喹啉酮,合成了 42 种新型类似物,并研究了它们的体外代谢稳定性和细胞毒性以及它们的体内药代动力学、基本行为药理学和对运动的影响。将氘掺入配体的甲氧基取代基通过改善代谢稳定性和生物利用度增加了它们的作用持续时间,同时保留了它们对 GABAAR α6 亚型的选择性。8b 被确定为具有显着改善的药代动力学特征的先导化合物。配体对地西泮不敏感的α6β2/3γ2 GABAARs 进行变构调节,并且对地西泮敏感的α1β2/3γ2 GABAARs 功能沉默,因此未检测到镇静作用。此外,这些类似物没有细胞毒性,这使它们成为治疗由 GABAAR α6β2/3γ2 亚型介导的 CNS 疾病的有趣候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号