当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-catalyzed asymmetric hydroboration of 1,3-enynes with pinacolborane to access chiral allenylboronates†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c8qo00167g Hui Leng Sang 1, 2, 3, 4 , Songjie Yu 1, 2, 3, 4 , Shaozhong Ge 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c8qo00167g Hui Leng Sang 1, 2, 3, 4 , Songjie Yu 1, 2, 3, 4 , Shaozhong Ge 1, 2, 3, 4
Affiliation
|
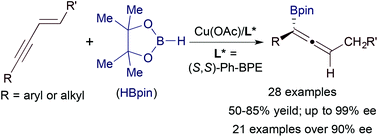
We report a copper-catalyzed enantioselective hydroboration of 1,3-enynes with the catalyst generated from Cu(OAc) and (S,S)-Ph-BPE. A wide range of alkyl- and aryl-substituted 1,3-enynes undergo this asymmetric hydroboration with pinacolborane, yielding the corresponding allenes in good yields with high to excellent enantioselectivities (up to 99% ee). This asymmetric transformation tolerates a variety of reactive groups, such as chloro, bromo, trifluoromethyl ether, siloxy, carboxylic ester and imido functionalities.
中文翻译:

频哪醇硼烷对铜催化的1,3-烯炔的不对称硼氢化反应以得到手性烯丙基硼酸酯†
我们报告了由Cu(OAc)和(S,S)-Ph-BPE生成的催化剂对1,3-炔烃的铜催化对映选择性氢硼化。各种各样的烷基和芳基取代的1,3-烯炔与频哪醇硼烷进行这种不对称硼氢化反应,以高收率和高至优异的对映选择性(高达99%ee)产生相应的异戊烯。这种不对称转变可耐受各种反应性基团,例如氯,溴,三氟甲基醚,甲硅烷氧基,羧酸酯和亚氨基官能团。
更新日期:2018-02-26
中文翻译:

频哪醇硼烷对铜催化的1,3-烯炔的不对称硼氢化反应以得到手性烯丙基硼酸酯†
我们报告了由Cu(OAc)和(S,S)-Ph-BPE生成的催化剂对1,3-炔烃的铜催化对映选择性氢硼化。各种各样的烷基和芳基取代的1,3-烯炔与频哪醇硼烷进行这种不对称硼氢化反应,以高收率和高至优异的对映选择性(高达99%ee)产生相应的异戊烯。这种不对称转变可耐受各种反应性基团,例如氯,溴,三氟甲基醚,甲硅烷氧基,羧酸酯和亚氨基官能团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号