当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible stapling of unprotected peptides via chemoselective methionine bis-alkylation/dealkylation†
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c7sc05109c Xiaodong Shi 1 , Rongtong Zhao 1 , Yixiang Jiang 1 , Hui Zhao 2 , Yuan Tian 3 , Yanhong Jiang 1 , Jingxu Li 1 , Weirong Qin 1 , Feng Yin 1 , Zigang Li 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-02-26 00:00:00 , DOI: 10.1039/c7sc05109c Xiaodong Shi 1 , Rongtong Zhao 1 , Yixiang Jiang 1 , Hui Zhao 2 , Yuan Tian 3 , Yanhong Jiang 1 , Jingxu Li 1 , Weirong Qin 1 , Feng Yin 1 , Zigang Li 1
Affiliation
|
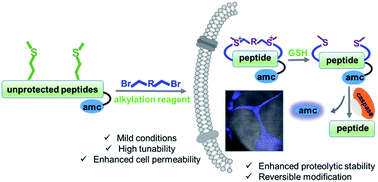
We have developed a general peptide macrocyclization strategy that involves a facile and chemoselective methionine bis-alkylation/dealkylation process. This method provides a straightforward and easy approach to generate cyclic peptides with tolerances of all amino acids (including Cys), variable loop sizes, and different linkers. The Met bis-alkylation we apply in this strategy yields two additional on-tether positive charges that could assist in the cellular uptake of the peptides. Notably, the bis-alkylated peptide could be reduced to release the original peptide both in vitro and within cellular environments. This strategy provides an intriguing and facile traceless post-peptide-synthesis modification with enhanced cellular uptakes. Peptides constructed with this method could be utilized to zero in on various protein targets or to achieve other goals, such as drug delivery.
中文翻译:

通过化学选择性蛋氨酸双烷基化/脱烷基化可逆地装订未受保护的肽†
我们开发了一种通用的肽大环化策略,涉及简单且化学选择性的甲硫氨酸双烷基化/脱烷基化过程。该方法提供了一种简单易行的方法来生成具有所有氨基酸(包括 Cys)、可变环大小和不同接头的环肽。我们在该策略中应用的 Met 双烷基化产生两个额外的系链正电荷,可以帮助细胞摄取肽。值得注意的是,双烷基化肽可以在体外和细胞环境中被还原以释放原始肽。该策略提供了一种有趣且简便的无痕肽合成后修饰,并增强了细胞的摄取。用这种方法构建的肽可用于将各种蛋白质靶标归零或实现其他目标,例如药物输送。
更新日期:2018-02-26
中文翻译:

通过化学选择性蛋氨酸双烷基化/脱烷基化可逆地装订未受保护的肽†
我们开发了一种通用的肽大环化策略,涉及简单且化学选择性的甲硫氨酸双烷基化/脱烷基化过程。该方法提供了一种简单易行的方法来生成具有所有氨基酸(包括 Cys)、可变环大小和不同接头的环肽。我们在该策略中应用的 Met 双烷基化产生两个额外的系链正电荷,可以帮助细胞摄取肽。值得注意的是,双烷基化肽可以在体外和细胞环境中被还原以释放原始肽。该策略提供了一种有趣且简便的无痕肽合成后修饰,并增强了细胞的摄取。用这种方法构建的肽可用于将各种蛋白质靶标归零或实现其他目标,例如药物输送。











































 京公网安备 11010802027423号
京公网安备 11010802027423号