Molecular Catalysis ( IF 4.6 ) Pub Date : 2018-02-24 , DOI: 10.1016/j.mcat.2018.02.005 Li Kang , Jin Zhang , Riguang Zhang , Lixia Ling , Baojun Wang
|
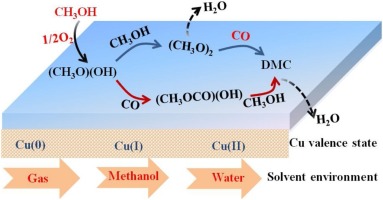
The formation mechanism and kinetics of dimethyl carbonate (DMC) via the oxidative carbonylation of methanol over Cu(II) catalyst have been investigated using density functional theory calculations under gas phase and solvent conditions. The results show that the preferred pathway of DMC formation in the gas phase is that via CO insertion into (CH3O)2 species, which is more favorable in kinetics than that via CO insertion into CH3O; the rate-limiting step of DMC formation is CO insertion into (CH3O)2. Meanwhile, the kinetic results of DMC formation in the liquid phase show that the solvent can improve the catalytic activity of Cu(II) catalyst towards DMC formation in a liquid-phase slurry, especially in water. Moreover, the comparisons among different valence state Cu catalysts for the most favorable pathway of DMC formation indicate that Cu valence state has a significant effect on the formation mechanism of DMC. The calculated results can provide a clue to finely tune the catalytic activity of DMC formation over Cu-based catalyst using the valence state and solvent environments under the realistic conditions.
中文翻译:

CuO催化剂上甲醇氧化羰基化为碳酸二甲酯的形成机理和动力学的研究:Cu价态和溶剂环境的影响
在气相和溶剂条件下,使用密度泛函理论计算研究了甲醇在Cu(II)催化剂上氧化羰基化形成碳酸二甲酯(DMC)的机理和动力学。结果表明,气相中DMC形成的优选途径是通过CO插入(CH 3 O)2物种,在动力学上比通过CO插入CH 3 O更好。DMC形成的限速步骤是将CO插入(CH 3 O)2。同时,液相中DMC形成的动力学结果表明,溶剂可以提高Cu(II)催化剂对液相浆液中DMC形成的催化活性,特别是在水中。此外,通过比较不同价态的Cu催化剂对DMC形成最有利途径的影响,发现Cu价态对DMC的形成机理具有重要影响。计算结果可以为在实际条件下使用化合价态和溶剂环境精细调节DMC形成对Cu基催化剂的催化活性提供线索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号