Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-02-24 , DOI: 10.1016/j.mcat.2018.02.013 Anangamohan Panja , Narayan Ch. Jana , Paula Brandão
|
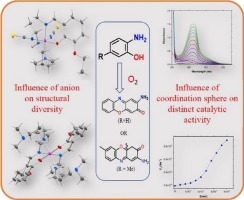
The present work reports the synthesis and structural characterizations of five new cobalt complexes (1–5) resulting from a N3O donor ligand, a Schiff base condensation product of N,N-dimethyldipropylenetriamine and 3-ethoxysalicylaldehyde, and their catalytic activity for the aerobic oxidation of various substrates, namely o-aminophenols and catechol. X-ray structural studies reveal that the Schiff base ligand can bind the metal centre either in the tetradentate fashion using all the available donor sites of the monoanionic deprotonated form (in 2 and 3) or in the tridentate fashion using the zwitterionic form of the Schiff base ligand with pendent quaternary amine nitrogen (in 1 and 4). Additionally, the monoanionic deprotonated ligand can bind the metal centre in the tridentate fashion (in 4 and 5) where the pendent tertiary amine nitrogen is involved in the intramolecular hydrogen bonding. All these adaptabilities associated with this triamine make it appealing candidate for exploration of the coordination chemistry with diverse structures. All complexes except compound 1 are efficient functional models for phenoxazinone synthase, and as expected the availability of labile sites at the first coordination sphere for the substrate, o-aminophenol, binding is the governing factor for higher catalytic activity in 2 and 3. Requisite of the hydrogen bond acceptor centre as well as proton abstraction centre at the second coordination sphere behind the facile oxidation of the substrate were also explored (reactivity of 1 versus 4 and 5). Moreover, the broader catalytic ability of these complexes was examined with substituted aminophenol and catechol as substrates, and the results were assembled and analysed. Furthermore, emphasis was given to get insight into the mechanistic pathway of functioning phenoxazinone synthase activity, well supported by the mass spectral study.
中文翻译:

阴离子和溶剂对钴不同配位化学的影响以及配位球对邻氨基苯酚仿生氧化的影响
本工作报告了由N 3 O供体配体,N,N-二甲基二丙烯三胺和3-乙氧基水杨醛的席夫碱缩合产物产生的5种新的钴配合物(1-5)的合成和结构表征,以及它们对苯的催化活性。各种底物,即邻氨基苯酚和邻苯二酚的好氧氧化。X射线结构研究表明,席夫碱配体可以使用单阴离子去质子化形式(在2和3中)的所有可用供体位点以四齿形式结合金属中心,或者使用席夫的两性离子形式以三齿形式结合金属中心。带有季胺氮原子的碱性配体(在1和4)。另外,单阴离子去质子化的配体可以以三齿方式(以4和5)结合金属中心,其中悬垂的叔胺氮参与分子内氢键。与三胺有关的所有这些适应性使其成为探索具有不同结构的配位化学的有吸引力的候选者。除化合物1外,所有复合物都是苯恶嗪酮合酶的有效功能模型,并且如预期的那样,底物邻氨基苯酚在第一个配位面上的不稳定位点的可用性是2和3中较高催化活性的控制因素。。氢键受体中心以及在所述衬底的所述容易氧化的后面的第二配位球质子抽象中心的必要还探讨(的反应1 对 4和5)。此外,以取代的氨基苯酚和邻苯二酚为底物考察了这些配合物的更广泛的催化能力,并对结果进行了组装和分析。此外,重点在于深入研究功能苯恶嗪酮合酶活性的机制途径,并得到了质谱研究的大力支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号