Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-02-24 , DOI: 10.1016/j.jcat.2018.01.034 Quanxing Zheng , Jiayi Xu , Bin Liu , Keith L. Hohn
|
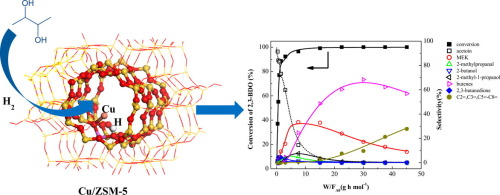
The reaction kinetics of 2,3-butanediol (2,3-BDO) and other key intermediates, including methyl ethyl ketone (MEK), 2-methylpropanal, acetoin, 2-butanol and 2-methyl-1-propanol, were investigated over acidic zeolites (ZSM-5, and Y-type zeolite), Cu/ZSM-5, Cu/Y and Cu/SiO2 to elucidate the roles of acid and metal sites in the process of hydrodeoxygenation of 2,3-BDO to butene. Hydrogenation and dehydrogenation reactions occur on Cu sites, while dehydration reactions take place on acid sites. At low space time, 2,3-BDO can readily be converted to acetoin by dehydrogenation over supported Cu catalysts, however, with increasing space time, the formed acetoin is hydrogenated back to 2,3-BDO. DFT (density functional theory) calculations suggest that the cage structure of Y zeolite allows the formation of larger Cu clusters, potentially blocking acid sites and preventing access of C4 alcohols (2-butanol, 2-methyl-1-propanol) to the acid sites for dehydration. This effect results in lower selectivity to butenes over Cu/Y than on Cu/ZSM-5.
中文翻译:

2,3-丁二醇催化转化为丁烯的机理研究
研究了2,3-丁二醇(2,3-BDO)与其他关键中间体的反应动力学,其中包括甲乙酮(MEK),2-甲基丙醛,丙酮,2-丁醇和2-甲基-1-丙醇。酸性沸石(ZSM-5和Y型沸石),Cu / ZSM-5,Cu / Y和Cu / SiO 2阐明酸和金属位点在2,3-BDO加氢脱氧为丁烯的过程中的作用。氢化和脱氢反应发生在Cu位,而脱水反应发生在酸性位。在低时空下,2,3-BDO可以通过在负载型Cu催化剂上进行脱氢反应而容易地转化为乙醛,但是,随着时空的增加,所形成的乙醛被氢化回2,3-BDO。DFT(密度泛函理论)计算表明,Y沸石的笼状结构允许形成较大的铜团簇,潜在地阻止酸位,并阻止C 4醇(2-丁醇,2-甲基-1-丙醇)进入酸中脱水部位。这种作用导致在Cu / Y上对丁烯的选择性比在Cu / ZSM-5上低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号