Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-02-24 , DOI: 10.1016/j.jinorgbio.2018.02.017 Ari Dwi Nugraheni , Chunguang Ren , Yorifumi Matsumoto , Satoshi Nagao , Masaru Yamanaka , Shun Hirota
|
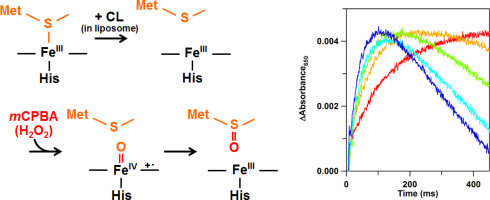
The Met80–heme iron bond of cytochrome c (cyt c) is cleaved by the interaction of cyt c with cardiolipin (CL) in membranes. The Met80 dissociation enhances the peroxidase activity of cyt c and triggers cyt c release from mitochondrion to the cytosol at the early stage of apoptosis. This paper demonstrates the selective oxidation of Met80 for the reaction of ferric cyt c with a peroxide, meta-chloroperbenzoic acid (mCPBA), in the presence of CL-containing liposomes by formation of a ferryl species (Compound I). After the reaction of cyt c with mCPBA in the presence of 1,2-dioloeyl-sn-glycero-3-phosphocholine (DOPC) liposomes containing CL, the electrospray ionization mass spectrum of the peptide fragments, obtained by digestion of cyt c with lysyl endopeptidase, exhibited a peak at m/z = 795.45; whereas, this peak was not observed for the peptide fragments obtained after the reaction in the presence of DOPC liposomes not containing CL. According to the tandem mass spectrum of the m/z = 795.45 peptide fragment, Met80 was modified with a 16 Da mass increase. The purified Met80-modified cyt c exhibited a peroxidase activity more than 5-fold higher than that of the unmodified protein. Transient absorption bands around 650 nm were generated by the reactions with mCPBA for ferric wild-type cyt c in the presence of CL-containing DOPC liposomes and ferric Y67F cyt c in the absence of liposomes. The formation and decomposition rates of the 650-nm absorption species increased and decreased, respectively, by increasing the mCPBA concentration in the reaction, indicating transient formation of Compound I.
中文翻译:

与过氧化物反应对细胞色素c中的蛋氨酸80进行氧化修饰
细胞色素c(cyt c)的Met80-血红素铁键通过cyt c与心磷脂(CL)在膜中的相互作用而被裂解。Met80的解离增强了cyt c的过氧化物酶活性,并触发了cyt c在细胞凋亡的早期从线粒体释放到细胞质中。本文演示Met80的用于铁细胞色素的反应选择性氧化Ç用过氧化物,间氯过苯甲酸(米CPBA),在通过形成ferryl物种(化合物I)的存在下对含CL-脂质体。cyt c与m CPBA在1,2-二油酰基-存在下反应后含有CL的sn-甘油3-磷酸胆碱(DOPC)脂质体,通过用赖氨酰内肽酶消化cyt c得到的肽片段的电喷雾电离质谱图,在m / z = 795.45处显示峰。相反,在不含CL的DOPC脂质体存在下,反应后得到的肽片段未观察到该峰。根据m / z = 795.45肽片段的串联质谱图,对Met80进行了修饰,质量增加了16 Da。纯化的Met80修饰的cyt c表现出的过氧化物酶活性是未修饰蛋白的5倍以上。通过与反应生成的650nm附近的瞬态吸收带米CPBA为三价铁的野生型细胞色素Ç在含有CL-DOPC脂质体和铁Y67F细胞色素的存在Ç在不存在脂质体。通过增加反应中的m CPBA浓度,分别增加和减少650 nm吸收物质的形成和分解速率,表明化合物I的瞬时形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号