Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineered red blood cells for capturing circulating tumor cells with high performance
Nanoscale ( IF 5.8 ) Pub Date : 2018-02-24 00:00:00 , DOI: 10.1039/c7nr08032h Dao-Ming Zhu 1, 2, 3, 4, 5 , Lei Wu 3, 4, 5, 6, 7 , Meng Suo 1, 2, 3, 4, 5 , Song Gao 1, 2, 3, 4, 5 , Wei Xie 1, 2, 3, 4, 5 , Ming-Hui Zan 1, 2, 3, 4, 5 , Ao Liu 1, 2, 3, 4, 5 , Bei Chen 1, 2, 3, 4, 5 , Wen-Tao Wu 1, 2, 3, 4, 5 , Li-Wei Ji 1, 2, 3, 4, 5 , Li-ben Chen 8, 9, 10, 11 , Hui-Ming Huang 1, 2, 3, 4, 5 , Shi-Shang Guo 1, 2, 3, 4, 5 , Wen-Feng Zhang 3, 4, 5, 6, 7 , Xing-Zhong Zhao 1, 2, 3, 4, 5 , Zhi-Jun Sun 3, 4, 5, 6, 7 , Wei Liu 1, 2, 3, 4, 5
Nanoscale ( IF 5.8 ) Pub Date : 2018-02-24 00:00:00 , DOI: 10.1039/c7nr08032h Dao-Ming Zhu 1, 2, 3, 4, 5 , Lei Wu 3, 4, 5, 6, 7 , Meng Suo 1, 2, 3, 4, 5 , Song Gao 1, 2, 3, 4, 5 , Wei Xie 1, 2, 3, 4, 5 , Ming-Hui Zan 1, 2, 3, 4, 5 , Ao Liu 1, 2, 3, 4, 5 , Bei Chen 1, 2, 3, 4, 5 , Wen-Tao Wu 1, 2, 3, 4, 5 , Li-Wei Ji 1, 2, 3, 4, 5 , Li-ben Chen 8, 9, 10, 11 , Hui-Ming Huang 1, 2, 3, 4, 5 , Shi-Shang Guo 1, 2, 3, 4, 5 , Wen-Feng Zhang 3, 4, 5, 6, 7 , Xing-Zhong Zhao 1, 2, 3, 4, 5 , Zhi-Jun Sun 3, 4, 5, 6, 7 , Wei Liu 1, 2, 3, 4, 5
Affiliation
|
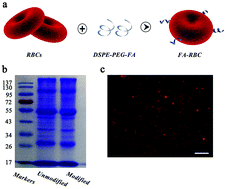
Filtration of circulating tumor cells (CTCs) in peripheral blood is of proven importance for early cancer diagnosis, treatment monitoring, metastasis diagnosis, and prognostic evaluation. However, currently available strategies for enriching CTCs, such as magnetic activated cell sorting (MACS), face serious problems with purity due to nonspecific interactions between beads and leukocytes in the process of capturing. In the present study, the tumor-targeting molecule folic acid (FA) and magnetic nanoparticles (MNPs) were coated on the surface of red blood cells (RBCs) by hydrophobic interaction and chemical conjugation, respectively. The resulting engineered RBCs rapidly adhered to CTCs and the obtained CTC–RBC conjugates were isolated in a magnetic field. After treatment with RBC lysis buffer and centrifugation, CTCs were released and captured. The duration of the entire process was less than three hours. Cell counting showed that the capture efficiency was above 90% and the purity of the obtained CTCs was higher than 75%. The performance of the proposed method exceeded that of MACS® beads (80% for capture efficiency and 20% for purity) under the same conditions. The obtained CTCs could be successfully re-cultured and proliferated in vitro. Our engineered RBCs have provided a novel method for enriching rare cells in the physiological environment.
中文翻译:

工程红细胞可高效捕获循环肿瘤细胞
外周血中循环肿瘤细胞(CTC)的过滤对早期癌症诊断,治疗监测,转移诊断和预后评估具有重要意义。然而,由于珠子和白细胞之间在捕获过程中的非特异性相互作用,诸如磁活化细胞分选(MACS)之类的当前可用的富集CTC的策略面临纯度方面的严重问题。在本研究中,肿瘤靶向分子叶酸(FA)和磁性纳米颗粒(MNPs)分别通过疏水作用和化学结合作用涂覆在红细胞(RBC)的表面上。产生的工程RBC迅速粘附到CTC上,并且在磁场中分离出获得的CTC-RBC共轭物。用RBC裂解缓冲液处理并离心后,释放并捕获了四氯化碳。整个过程的持续时间少于三个小时。细胞计数表明捕获效率高于90%,所得四氯化碳的纯度高于75%。在相同条件下,所提出方法的性能超过了MACS®磁珠的性能(捕获效率为80%,纯度为20%)。获得的四氯化碳可以成功地再培养和增殖体外。我们的工程红细胞提供了一种在生理环境中富集稀有细胞的新方法。
更新日期:2018-02-24
中文翻译:

工程红细胞可高效捕获循环肿瘤细胞
外周血中循环肿瘤细胞(CTC)的过滤对早期癌症诊断,治疗监测,转移诊断和预后评估具有重要意义。然而,由于珠子和白细胞之间在捕获过程中的非特异性相互作用,诸如磁活化细胞分选(MACS)之类的当前可用的富集CTC的策略面临纯度方面的严重问题。在本研究中,肿瘤靶向分子叶酸(FA)和磁性纳米颗粒(MNPs)分别通过疏水作用和化学结合作用涂覆在红细胞(RBC)的表面上。产生的工程RBC迅速粘附到CTC上,并且在磁场中分离出获得的CTC-RBC共轭物。用RBC裂解缓冲液处理并离心后,释放并捕获了四氯化碳。整个过程的持续时间少于三个小时。细胞计数表明捕获效率高于90%,所得四氯化碳的纯度高于75%。在相同条件下,所提出方法的性能超过了MACS®磁珠的性能(捕获效率为80%,纯度为20%)。获得的四氯化碳可以成功地再培养和增殖体外。我们的工程红细胞提供了一种在生理环境中富集稀有细胞的新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号