Catalysis Today ( IF 5.2 ) Pub Date : 2018-02-24 , DOI: 10.1016/j.cattod.2018.02.042 Kristof De Wispelaere , Juan S. Martínez-Espín , Max J. Hoffmann , Stian Svelle , Unni Olsbye , Thomas Bligaard
|
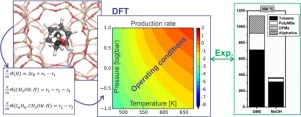
In methanol-to-hydrocarbon chemistry, methanol and dimethyl ether (DME) can act as methylating agents. Therefore, we focus on the different reactivity of methanol and DME towards benzene methylation in H-ZSM-5 at operating conditions by combining first principles microkinetic modeling and experiments. Methylation reactions are known to follow either a concerted reaction path or a stepwise mechanism going through a framework-bound methoxide. By constructing a DFT based microkinetic model including the concerted and stepwise reactions, product formation rates can be calculated at conditions that closely mimic the experimentally applied conditions. Trends in measured rates are relatively well reproduced by our DFT based microkinetic model. We find that benzene methylation with DME is faster than with methanol but the difference decreases with increasing temperature. At low temperatures, the concerted mechanism dominates, however at higher temperatures and low pressures the mechanism shifts to the stepwise pathway. This transition occurs at lower temperatures for methanol than for DME, resulting in smaller reactivity differences between methanol and DME at high temperature. Our theory-experiment approach shows that the widely assumed rate law with zeroth and first order in oxygenate and hydrocarbon partial pressure is not generally applicable and depends on the applied temperature, pressure and feed composition.
中文翻译:

从第一原理微观动力学模型和实验中了解在操作条件下甲醇和二甲醚进行沸石催化的苯甲基化反应
在甲醇制烃化学中,甲醇和二甲醚(DME)可以充当甲基化剂。因此,我们通过结合第一原理微观动力学建模和实验,重点研究了在操作条件下,甲醇和DME对H-ZSM-5中苯甲基化的不同反应性。已知甲基化反应遵循一致的反应路径或通过框架结合的甲醇盐的逐步机理。通过构建包括协同反应和逐步反应在内的基于DFT的微动力学模型,可以在与实验应用条件极为相似的条件下计算出产物形成速率。我们基于DFT的微动力学模型相对较好地再现了测量速率的趋势。我们发现用DME进行的苯甲基化比使用甲醇进行的甲基化要快,但差异随温度的升高而减小。在低温下,协调机构占主导地位,但是在较高温度和低压下,该机构转向阶梯式路径。甲醇的这种转变发生在比DME更低的温度下,从而导致甲醇和DME在高温下的反应性差异更小。我们的理论实验方法表明,氧合剂和烃分压中零级和一阶的普遍假定的速率定律通常不适用,并且取决于所施加的温度,压力和进料组成。甲醇的这种转变发生在比DME更低的温度下,从而导致甲醇和DME在高温下的反应性差异更小。我们的理论实验方法表明,氧合剂和烃分压中零级和一阶的普遍假定的速率定律通常不适用,并且取决于所施加的温度,压力和进料组成。甲醇的这种转变发生在比DME更低的温度下,从而导致甲醇和DME在高温下的反应性差异更小。我们的理论实验方法表明,广泛采用的含氧量和烃分压中零级和一级的速率定律通常不适用,并且取决于所施加的温度,压力和进料组成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号