Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TiO 2 as a catalyst for hydrogen production from hydrogen-iodide in thermo-chemical water-splitting sulfur-iodine cycle
Fuel ( IF 6.7 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.fuel.2018.02.130 Amit Singhania , Ashok N. Bhaskarwar
Fuel ( IF 6.7 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.fuel.2018.02.130 Amit Singhania , Ashok N. Bhaskarwar
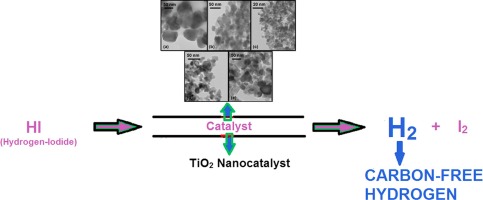
|
Abstract In this work, TiO 2 nanoparticles have been prepared by the sol–gel (at different calcination temperatures) and solution-combustion method for hydrogen-iodide decomposition in thermo-chemical water-splitting sulfur-iodine (SI) cycle for hydrogen production. The sol-gel method derived TiO 2 (TiO 2 -SGM-300 and TiO 2 -SGM-500) provide smaller nanoparticles as compared to the solution-combustion (TiO 2 -SCM). TEM revealed a particle size of around 4–5 nm of TiO 2 -SGM-300. XRD and Raman confirmed that TiO 2 -SGM-300 and TiO 2 -SGM-500 exhibited pure anatase phase, whereas a small amount of rutile phase was observed in TiO 2 -SGM-700 and TiO 2 -SCM samples. It is found that with increase in calcination temperatures during sol-gel method, the average particle size of TiO 2 increases and specific surface area decreases. Commercial TiO 2 (Degussa P-25) was used for the comparison purpose. As far as the author knows, TiO 2 has been used here for the first time for hydrogen-iodide decomposition. The hydrogen-iodide decomposition experiments were carried out in a vertical-fixed bed quartz reactor at a WHSV of 12.9 h −1 under atmospheric pressure. The order of catalytic activity was as follows: TiO 2 -SGM-300 > TiO 2 -SCM > TiO 2 -COMM (commercial). Also, it was observed that the hydrogen-iodide conversion decreases with increase in calcination temperatures of TiO 2 during sol–gel method. Their activity was as follows: TiO 2 -SGM-300 > TiO 2 -SGM-500 > TiO 2 -SGM-700. TiO 2 -SGM-300 catalyst also showed a reasonable time-on-stream stability of 6 h for hydrogen-iodide decomposition. The apparent activation energy of TiO 2 -SGM-300 is found to be 72.29 kJ mol −1 . This shows that the TiO 2 has a potential of generating hydrogen from hydrogen iodide in SI cycle. This can further be explored as a catalyst support using some non-precious and precious metal catalysts for hydrogen-iodide decomposition.
中文翻译:

TiO 2 作为热化学水分解硫碘循环中碘化氢制氢的催化剂
摘要 在这项工作中,通过溶胶-凝胶法(在不同的煅烧温度下)和溶液燃烧法制备了TiO 2 纳米颗粒,用于热化学水分解硫碘(SI)循环中的碘化氢分解制氢。与溶液燃烧法 (TiO 2 -SCM) 相比,溶胶-凝胶法衍生的 TiO 2 (TiO 2 -SGM-300 和 TiO 2 -SGM-500) 提供了更小的纳米颗粒。TEM 显示 TiO 2 -SGM-300 的粒径约为 4-5 nm。XRD和Raman证实TiO 2 -SGM-300和TiO 2 -SGM-500呈现纯锐钛矿相,而在TiO 2 -SGM-700和TiO 2 -SCM样品中观察到少量金红石相。发现溶胶-凝胶法随着煅烧温度的升高,TiO 2 的平均粒径增大,比表面积减小。商业TiO 2 (Degussa P-25)用于比较目的。据笔者所知,TiO 2 在这里是首次用于碘化氢分解。碘化氢分解实验在大气压下以 12.9 h -1 的 WHSV 垂直固定床石英反应器进行。催化活性的顺序如下:TiO 2 -SGM-300 > TiO 2 -SCM > TiO 2 -COMM(商业)。此外,据观察,在溶胶-凝胶法中,随着 TiO 2 煅烧温度的升高,碘化氢转化率降低。它们的活性如下:TiO 2 -SGM-300 > TiO 2 -SGM-500 > TiO 2 -SGM-700。对于碘化氢分解,TiO 2 -SGM-300 催化剂还显示出合理的 6 小时运行时间稳定性。发现 TiO 2 -SGM-300 的表观活化能为 72。29 kJ 摩尔 -1 。这表明TiO 2 具有在SI循环中由碘化氢产生氢气的潜力。这可以进一步探索作为催化剂载体,使用一些非贵金属和贵金属催化剂进行碘化氢分解。
更新日期:2018-06-01
中文翻译:

TiO 2 作为热化学水分解硫碘循环中碘化氢制氢的催化剂
摘要 在这项工作中,通过溶胶-凝胶法(在不同的煅烧温度下)和溶液燃烧法制备了TiO 2 纳米颗粒,用于热化学水分解硫碘(SI)循环中的碘化氢分解制氢。与溶液燃烧法 (TiO 2 -SCM) 相比,溶胶-凝胶法衍生的 TiO 2 (TiO 2 -SGM-300 和 TiO 2 -SGM-500) 提供了更小的纳米颗粒。TEM 显示 TiO 2 -SGM-300 的粒径约为 4-5 nm。XRD和Raman证实TiO 2 -SGM-300和TiO 2 -SGM-500呈现纯锐钛矿相,而在TiO 2 -SGM-700和TiO 2 -SCM样品中观察到少量金红石相。发现溶胶-凝胶法随着煅烧温度的升高,TiO 2 的平均粒径增大,比表面积减小。商业TiO 2 (Degussa P-25)用于比较目的。据笔者所知,TiO 2 在这里是首次用于碘化氢分解。碘化氢分解实验在大气压下以 12.9 h -1 的 WHSV 垂直固定床石英反应器进行。催化活性的顺序如下:TiO 2 -SGM-300 > TiO 2 -SCM > TiO 2 -COMM(商业)。此外,据观察,在溶胶-凝胶法中,随着 TiO 2 煅烧温度的升高,碘化氢转化率降低。它们的活性如下:TiO 2 -SGM-300 > TiO 2 -SGM-500 > TiO 2 -SGM-700。对于碘化氢分解,TiO 2 -SGM-300 催化剂还显示出合理的 6 小时运行时间稳定性。发现 TiO 2 -SGM-300 的表观活化能为 72。29 kJ 摩尔 -1 。这表明TiO 2 具有在SI循环中由碘化氢产生氢气的潜力。这可以进一步探索作为催化剂载体,使用一些非贵金属和贵金属催化剂进行碘化氢分解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号