Journal of Power Sources ( IF 8.1 ) Pub Date : 2018-02-22 , DOI: 10.1016/j.jpowsour.2018.02.021 Hamdi Ben Yahia , Rachid Essehli , Ruhul Amin , Khalid Boulahya , Toyoki Okumura , Ilias Belharouak
|
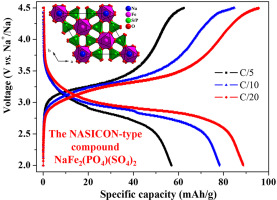
The compound NaFe2(PO4)(SO4)2 is successfully synthesized via a solid state reaction route and its crystal structure is determined using powder X-ray diffraction data. NaFe2(PO4)(SO4)2 phase is also characterized by cyclic voltammetry, galvanostatic cycling and electrochemical impedance spectroscopy. NaFe2(PO4)(SO4)2 crystallizes with the well-known NASICON-type structure. SAED and HRTEM experiments confirm the structural model, and no ordering between the PO4−3 and SO4−2 polyanions is detected. The electrochemical tests indicate that NaFe2(PO4)(SO4)2 is a 3 V sodium intercalating cathode. The electrical conductivity is relatively low (2.2 × 10−6 Scm−1 at 200 °C) and the obtained activation energy is ∼0.60eV. The GITT experiments indicate that the diffusivity values are in the range of 10−11-10−12 cm2/s within the measured sodium concentrations.
中文翻译:

钠插在磷酸亚铁阴极NaFe 2(PO 4)(SO 4)2中
通过固态反应路线成功合成了化合物NaFe 2(PO 4)(SO 4)2,并使用粉末X射线衍射数据确定了其晶体结构。NaFe 2(PO 4)(SO 4)2相的特征还在于循环伏安法,恒电流循环和电化学阻抗谱。NaFe 2(PO 4)(SO 4)2以众所周知的NASICON型结构结晶。SAED和HRTEM实验证实了结构模型,并且PO 4 -3和SO 4之间没有排序检测到-2个聚阴离子。电化学测试表明NaFe 2(PO 4)(SO 4)2是3 V钠嵌入阴极。电导率相对较低(200°C下为2.2×10 -6 Scm -1),并且获得的活化能为〜0.60eV。GITT实验表明,在测得的钠浓度范围内,扩散率值在10 -11 -10 -12 cm 2 / s的范围内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号