当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures and Protonation States of Hydrophilic–Cationic Diblock Copolymers and Their Binding with Plasmid DNA
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1021/acs.jpcb.7b07902 Seyoung Jung 1 , Timothy P. Lodge 1, 2 , Theresa M. Reineke 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1021/acs.jpcb.7b07902 Seyoung Jung 1 , Timothy P. Lodge 1, 2 , Theresa M. Reineke 2
Affiliation
|
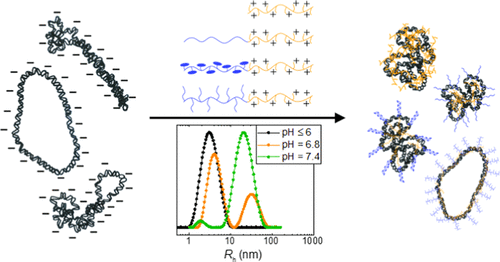
Complexation between plasmid DNA (pDNA) and a set of diblock copolymers, each with one cationic block and one hydrophilic, charge-neutral block, is examined. A range of hydrophilic block structures are explored, whereas the cationic block is fixed as poly(N-(2-aminoethyl) methacrylamide) (PAEMA) with a degree of polymerization of 60 ± 3. The hydrophilic blocks include poly(ethylene glycol) (PEG45), poly(oligo(ethylene glycol) methyl ether methacrylate) (P(OEGMA)51), and poly(2-deoxy-2-methacrylamido glucopyranose) (PMAG52). The numbers represent the degrees of polymerization and are chosen such that the diblock contour lengths are similar (37 ± 2 nm). The three diblock copolymers and a homopolycation control, PAEMA59, are compared with respect to their state of dissolution in aqueous environments, as well as their complexation with pDNA. The diblock copolymers are found to partially aggregate as pH increases above 6, whereas each separate block generally dissolves well over a wide pH range. The hydrophilic block proves to be a critical parameter in determining the structures of pDNA–diblock complexes. When the molar ratio of polycation amines to pDNA phosphates (i.e., N/P) is less than 1, a bulkier hydrophilic block leads to larger resulting complexes. As more polycations are added to the system (N/P > 1.5), colloidal stability becomes an important factor, making more water-soluble systems stabilize at smaller sizes. Further, the charge density effect on the binding thermodynamics is elucidated via calorimetric measurements. P(OEGMA)51-b-PAEMA60 exhibits a greater amount of endothermic pDNA binding per charged amine at higher pH, implying that lower cationic charge density promotes more phosphate pairing per amine on average. Also, the colloidal stability and the circular dichroism spectral evolution of the pDNA–PAEMA59 complexes are dependent on pH, showing noticeable differences between pH = 6.0 vs 7.4. To summarize, controlling the solution pH may be crucial in pDNA–polycation complexation, as it impacts polycation solubility, binding characteristics, and the final complex properties. The findings reported herein should aid researchers in drawing more rigorous structure–function correlations in the field of polymeric gene delivery.
中文翻译:

亲水性阳离子二嵌段共聚物的结构和质子化态及其与质粒DNA的结合
检查了质粒DNA(pDNA)和一组双嵌段共聚物之间的复合情况,每个双嵌段共聚物都有一个阳离子嵌段和一个亲水的电荷中性嵌段。探索了一系列亲水性嵌段结构,而阳离子嵌段则固定为聚(N聚合度为60±3的-(2-氨基乙基)甲基丙烯酰胺)(PAEMA)。亲水性嵌段包括聚(乙二醇)(PEG45),聚(低聚(乙二醇)甲基丙烯酸甲酯)(P(OEGMA) 51),和聚(2-脱氧-2-甲基丙烯酰胺基吡喃葡萄糖)(PMAG52)。数字代表聚合度,其选择应使二嵌段轮廓长度相似(37±2 nm)。比较了这三种二嵌段共聚物和均聚阳离子对照物PAEMA59在水性环境中的溶解状态以及与pDNA的络合情况。发现当pH增加到6以上时,二嵌段共聚物部分聚集,而每个单独的嵌段通常在宽的pH范围内很好地溶解。亲水性嵌段被证明是确定pDNA-二嵌段复合物结构的关键参数。当聚阳离子胺与pDNA磷酸盐的摩尔比(即N / P)小于1时,较大的亲水性嵌段会导致形成较大的配合物。随着向系统中添加更多的聚阳离子(N / P> 1.5),胶体稳定性成为重要因素,从而使更多的水溶性系统在较小的尺寸下稳定下来。此外,通过量热测量阐明了电荷密度对结合热力学的影响。P(OEGMA)51- 使更多的水溶性系统稳定在较小的尺寸上。此外,通过量热测量阐明了电荷密度对结合热力学的影响。P(OEGMA)51- 使更多的水溶性系统稳定在较小的尺寸上。此外,通过量热测量阐明了电荷密度对结合热力学的影响。P(OEGMA)51-b -PAEMA60在较高的pH值下,每个带电荷的胺均表现出更大的吸热pDNA结合力,这意味着较低的阳离子电荷密度平均可促进每个胺中更多的磷酸盐配对。同样,pDNA–PAEMA59复合物的胶体稳定性和圆二色性光谱演化也取决于pH值,表明pH = 6.0与7.4之间存在显着差异。综上所述,控制溶液的pH值可能对pDNA-聚阳离子的络合至关重要,因为它会影响聚阳离子的溶解度,结合特性和最终的络合物特性。本文报道的发现应有助于研究人员在聚合基因递送领域中得出更严格的结构-功能相关性。
更新日期:2018-02-23
中文翻译:

亲水性阳离子二嵌段共聚物的结构和质子化态及其与质粒DNA的结合
检查了质粒DNA(pDNA)和一组双嵌段共聚物之间的复合情况,每个双嵌段共聚物都有一个阳离子嵌段和一个亲水的电荷中性嵌段。探索了一系列亲水性嵌段结构,而阳离子嵌段则固定为聚(N聚合度为60±3的-(2-氨基乙基)甲基丙烯酰胺)(PAEMA)。亲水性嵌段包括聚(乙二醇)(PEG45),聚(低聚(乙二醇)甲基丙烯酸甲酯)(P(OEGMA) 51),和聚(2-脱氧-2-甲基丙烯酰胺基吡喃葡萄糖)(PMAG52)。数字代表聚合度,其选择应使二嵌段轮廓长度相似(37±2 nm)。比较了这三种二嵌段共聚物和均聚阳离子对照物PAEMA59在水性环境中的溶解状态以及与pDNA的络合情况。发现当pH增加到6以上时,二嵌段共聚物部分聚集,而每个单独的嵌段通常在宽的pH范围内很好地溶解。亲水性嵌段被证明是确定pDNA-二嵌段复合物结构的关键参数。当聚阳离子胺与pDNA磷酸盐的摩尔比(即N / P)小于1时,较大的亲水性嵌段会导致形成较大的配合物。随着向系统中添加更多的聚阳离子(N / P> 1.5),胶体稳定性成为重要因素,从而使更多的水溶性系统在较小的尺寸下稳定下来。此外,通过量热测量阐明了电荷密度对结合热力学的影响。P(OEGMA)51- 使更多的水溶性系统稳定在较小的尺寸上。此外,通过量热测量阐明了电荷密度对结合热力学的影响。P(OEGMA)51- 使更多的水溶性系统稳定在较小的尺寸上。此外,通过量热测量阐明了电荷密度对结合热力学的影响。P(OEGMA)51-b -PAEMA60在较高的pH值下,每个带电荷的胺均表现出更大的吸热pDNA结合力,这意味着较低的阳离子电荷密度平均可促进每个胺中更多的磷酸盐配对。同样,pDNA–PAEMA59复合物的胶体稳定性和圆二色性光谱演化也取决于pH值,表明pH = 6.0与7.4之间存在显着差异。综上所述,控制溶液的pH值可能对pDNA-聚阳离子的络合至关重要,因为它会影响聚阳离子的溶解度,结合特性和最终的络合物特性。本文报道的发现应有助于研究人员在聚合基因递送领域中得出更严格的结构-功能相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号