当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Critical Nuclei Size, Rate, and Activation Energy of H2 Gas Nucleation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-23 , DOI: 10.1021/jacs.7b13457 Sean R. German 1 , Martin A. Edwards 1 , Hang Ren 1 , Henry S. White 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-23 , DOI: 10.1021/jacs.7b13457 Sean R. German 1 , Martin A. Edwards 1 , Hang Ren 1 , Henry S. White 1
Affiliation
|
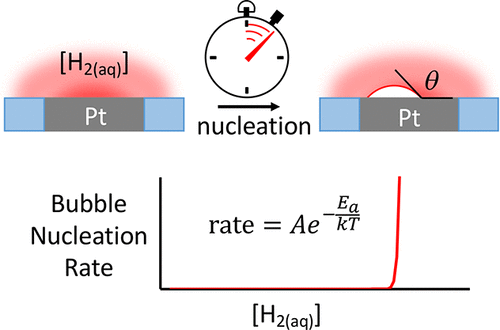
Electrochemical measurements of the nucleation rate of individual H2 bubbles at the surface of Pt nanoelectrodes (radius = 7-41 nm) are used to determine the critical size and geometry of H2 nuclei leading to stable bubbles. Precise knowledge of the H2 concentration at the electrode surface, CH2surf, is obtained by controlled current reduction of H+ in a H2SO4 solution. Induction times of single-bubble nucleation events are measured by stepping the current, to control CH2surf, while monitoring the voltage. We find that gas nucleation follows a first-order rate process; a bubble spontaneously nucleates after a stochastic time delay, as indicated by a sudden voltage spike that results from impeded transport of H+ to the electrode. Hundreds of individual induction times, at different applied currents and using different Pt nanoelectrodes, are used to characterize the kinetics of phase nucleation. The rate of bubble nucleation increases by four orders of magnitude (0.3-2000 s-1) over a very small relative change in CH2surf (0.21-0.26 M, corresponding to a ∼0.025 V increase in driving force). Classical nucleation theory yields thermodynamic radii of curvature for critical nuclei of 4.4 to 5.3 nm, corresponding to internal pressures of 330 to 270 atm, and activation energies for nuclei formation of 14 to 26 kT, respectively. The dependence of nucleation rate on H2 concentration indicates that nucleation occurs by a heterogeneous mechanism, where the nuclei have a contact angle of ∼150° with the electrode surface and contain between 35 and 55 H2 molecules.
中文翻译:

H2 气体成核的临界核尺寸、速率和活化能
Pt 纳米电极(半径 = 7-41 nm)表面单个 H2 气泡的成核速率的电化学测量用于确定导致稳定气泡的 H2 核的临界尺寸和几何形状。通过 H2SO4 溶液中 H+ 的受控电流还原,可以获得电极表面 H2 浓度的精确信息 CH2surf。单气泡成核事件的诱导时间是通过步进电流来测量的,以控制 CH2surf,同时监测电压。我们发现气体成核遵循一阶速率过程;气泡在随机时间延迟后自发成核,如由 H+ 向电极传输受阻导致的突然电压尖峰所表明。数百个单独的感应时间,在不同的施加电流下,使用不同的 Pt 纳米电极,用于表征相成核的动力学。在 CH2surf 的非常小的相对变化(0.21-0.26 M,对应于驱动力增加 0.025 V 时),气泡成核速率增加了四个数量级(0.3-2000 s-1)。经典成核理论得出临界核的热力学曲率半径为 4.4 至 5.3 nm,对应于 330 至 270 个大气压的内部压力,核形成的活化能分别为 14 至 26 kT。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。3-2000 s-1) 在 CH2surf 的非常小的相对变化(0.21-0.26 M,对应于 ∼0.025 V 的驱动力增加)。经典成核理论得出临界核的热力学曲率半径为 4.4 至 5.3 nm,对应于 330 至 270 个大气压的内部压力,核形成的活化能分别为 14 至 26 kT。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。3-2000 s-1) 在 CH2surf 的非常小的相对变化(0.21-0.26 M,对应于 ∼0.025 V 的驱动力增加)。经典成核理论得出临界核的热力学曲率半径为 4.4 至 5.3 nm,对应于 330 至 270 个大气压的内部压力,核形成的活化能分别为 14 至 26 kT。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。对应于 330 至 270 个大气压的内部压力,以及 14 至 26 kT 的成核活化能。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。对应于 330 至 270 个大气压的内部压力,以及 14 至 26 kT 的成核活化能。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。
更新日期:2018-02-23
中文翻译:

H2 气体成核的临界核尺寸、速率和活化能
Pt 纳米电极(半径 = 7-41 nm)表面单个 H2 气泡的成核速率的电化学测量用于确定导致稳定气泡的 H2 核的临界尺寸和几何形状。通过 H2SO4 溶液中 H+ 的受控电流还原,可以获得电极表面 H2 浓度的精确信息 CH2surf。单气泡成核事件的诱导时间是通过步进电流来测量的,以控制 CH2surf,同时监测电压。我们发现气体成核遵循一阶速率过程;气泡在随机时间延迟后自发成核,如由 H+ 向电极传输受阻导致的突然电压尖峰所表明。数百个单独的感应时间,在不同的施加电流下,使用不同的 Pt 纳米电极,用于表征相成核的动力学。在 CH2surf 的非常小的相对变化(0.21-0.26 M,对应于驱动力增加 0.025 V 时),气泡成核速率增加了四个数量级(0.3-2000 s-1)。经典成核理论得出临界核的热力学曲率半径为 4.4 至 5.3 nm,对应于 330 至 270 个大气压的内部压力,核形成的活化能分别为 14 至 26 kT。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。3-2000 s-1) 在 CH2surf 的非常小的相对变化(0.21-0.26 M,对应于 ∼0.025 V 的驱动力增加)。经典成核理论得出临界核的热力学曲率半径为 4.4 至 5.3 nm,对应于 330 至 270 个大气压的内部压力,核形成的活化能分别为 14 至 26 kT。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。3-2000 s-1) 在 CH2surf 的非常小的相对变化(0.21-0.26 M,对应于 ∼0.025 V 的驱动力增加)。经典成核理论得出临界核的热力学曲率半径为 4.4 至 5.3 nm,对应于 330 至 270 个大气压的内部压力,核形成的活化能分别为 14 至 26 kT。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。对应于 330 至 270 个大气压的内部压力,以及 14 至 26 kT 的成核活化能。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。对应于 330 至 270 个大气压的内部压力,以及 14 至 26 kT 的成核活化能。成核速率对 H2 浓度的依赖性表明成核是通过异质机制发生的,其中原子核与电极表面的接触角约为 150°,包含 35 到 55 个 H2 分子。










































 京公网安备 11010802027423号
京公网安备 11010802027423号