Virology ( IF 2.8 ) Pub Date : 2018-02-23 , DOI: 10.1016/j.virol.2018.01.021 Kenta Okamoto , Naoyuki Miyazaki , Hemanth K.N. Reddy , Max F. Hantke , Filipe R.N.C. Maia , Daniel S.D. Larsson , Chantal Abergel , Jean-Michel Claverie , Janos Hajdu , Kazuyoshi Murata , Martin Svenda
|
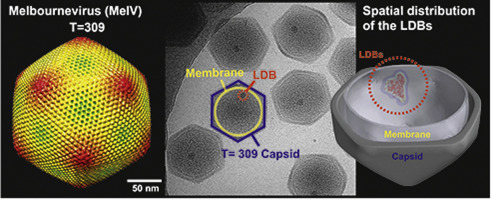
Nucleocytoplasmic large DNA viruses (NCLDVs) blur the line between viruses and cells. Melbournevirus (MelV, family Marseilleviridae) belongs to a new family of NCLDVs. Here we present an electron cryo-microscopy structure of the MelV particle, with the large triangulation number T = 309 constructed by 3080 pseudo-hexagonal capsomers. The most distinct feature of the particle is a large and dense body (LDB) consistently found inside all particles. Electron cryo-tomography of 147 particles shows that the LDB is preferentially located in proximity to the probable lipid bilayer. The LDB is 30 nm in size and its density matches that of a genome/protein complex. The observed LDB reinforces the structural complexity of MelV, setting it apart from other NCLDVs.
中文翻译:

马赛病毒科病毒颗粒的Cryo-EM结构揭示了较大的内部微装配
核胞质大DNA病毒(NCLDV)模糊了病毒和细胞之间的界线。墨尔本病毒(MelV,马赛病毒科)属于NCLDV的新家族。在这里,我们介绍了MelV粒子的电子冷冻显微镜结构,其大的三角剖分数T = 309由3080个伪六边形capsomers构成。粒子的最明显特征是在所有粒子内部始终存在的大型且密集的物体(LDB)。147个粒子的电子冷冻断层扫描显示,LDB优先位于可能的脂质双层附近。LDB的大小为30 nm,其密度与基因组/蛋白质复合物的密度相匹配。观察到的LDB增强了MelV的结构复杂性,使其与其他NCLDV区别开来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号