当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic asymmetric synthesis of compounds bearing both isoxazole and pyrazole moieties via 1,6-addition of pyrazol-5-ones to 3-methyl-4-nitro-5-alkenylisoxazoles†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1039/c8qo00084k Feng Li 1, 2, 3, 4, 5 , Wenlong Pei 1, 2, 3, 4, 5 , Jingjing Wang 1, 2, 3, 4, 5 , Jie Liu 1, 2, 3, 4, 5 , Juan Wang 1, 2, 3, 4, 5 , Ming-liang Zhang 1, 2, 3, 4, 5 , Zhiming Chen 5, 6, 7, 8 , Lantao Liu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1039/c8qo00084k Feng Li 1, 2, 3, 4, 5 , Wenlong Pei 1, 2, 3, 4, 5 , Jingjing Wang 1, 2, 3, 4, 5 , Jie Liu 1, 2, 3, 4, 5 , Juan Wang 1, 2, 3, 4, 5 , Ming-liang Zhang 1, 2, 3, 4, 5 , Zhiming Chen 5, 6, 7, 8 , Lantao Liu 1, 2, 3, 4, 5
Affiliation
|
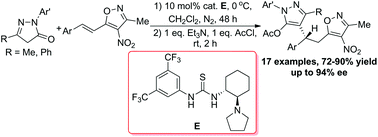
An efficient one-pot asymmetric synthesis of chiral compounds containing both isoxazole and pyrazole moieties has been developed. The bifunctional thiourea catalyzed asymmetric 1,6-addition of pyrazol-5-ones to 3-methyl-4-nitro-5-alkenylisoxazoles provided the corresponding acetylated chiral heterocycles after in situ treatment with AcCl/Et3N. The corresponding products were generated in good yields (72–90%) with high enantioselectivities (83–94% ee).
中文翻译:

通过吡唑-5-酮与3-甲基-4-硝基-5-烯 基异恶唑的1,6-加成反应,合成带有异恶唑和吡唑部分的化合物的有机催化不对称合成†
已经开发了一种高效的一锅不对称合成同时含有异恶唑和吡唑部分的手性化合物的方法。在用AcCl / Et 3 N原位处理后,双官能硫脲催化的吡唑-5-酮与3-甲基-4-硝基-5-链烯基异唑的不对称1,6-加成反应提供了相应的乙酰化手性杂环。具有高对映选择性(83-94%ee)的高收率(72-90%)。
更新日期:2018-02-23
中文翻译:

通过吡唑-5-酮与3-甲基-4-硝基-5-烯 基异恶唑的1,6-加成反应,合成带有异恶唑和吡唑部分的化合物的有机催化不对称合成†
已经开发了一种高效的一锅不对称合成同时含有异恶唑和吡唑部分的手性化合物的方法。在用AcCl / Et 3 N原位处理后,双官能硫脲催化的吡唑-5-酮与3-甲基-4-硝基-5-链烯基异唑的不对称1,6-加成反应提供了相应的乙酰化手性杂环。具有高对映选择性(83-94%ee)的高收率(72-90%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号