当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic combustion of diesel soot over Fe and Ag-doped manganese oxides: role of heteroatoms in the catalytic performances†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1039/c8cy00077h Yasutaka Kuwahara 1, 2, 3, 4, 5 , Akihiro Fujibayashi 1, 2, 3, 4, 5 , Hiroki Uehara 1, 2, 3, 4, 5 , Kohsuke Mori 1, 2, 3, 4, 5 , Hiromi Yamashita 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1039/c8cy00077h Yasutaka Kuwahara 1, 2, 3, 4, 5 , Akihiro Fujibayashi 1, 2, 3, 4, 5 , Hiroki Uehara 1, 2, 3, 4, 5 , Kohsuke Mori 1, 2, 3, 4, 5 , Hiromi Yamashita 1, 2, 3, 4, 5
Affiliation
|
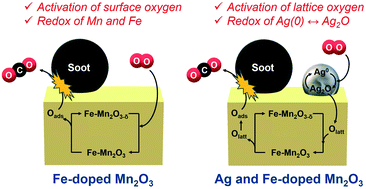
Fe-Doped manganese oxide catalysts were prepared by the citrate method and their catalytic activities in soot combustion were examined in air under tight contact mode. Among the catalysts tested, 12 mol% of Fe-doped α-Mn2O3 exhibited the highest activity, giving a soot combustion temperature (T50) of 328 °C, which is comparable to that previously reported for rare-earth-based catalysts, while pure α-Mn2O3 gave a T50 value of 339 °C. Comprehensive analyses by means of X-ray diffraction (XRD), specific surface area measurement, oxygen release rate measurement, X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure (XAFS) measurements revealed that the improved activity was due to i) an increased specific surface area and ii) an improved oxygen release rate involving reactive surface-adsorbed oxygen species. In situ XAFS measurements suggested that the interaction between Mn and Fe facilitated the activation of the Mn–O–Fe bond and increased the proportion of reactive oxygen species, thereby improving the redox properties of the catalysts. Furthermore, simultaneous doping of Ag and Fe into Mn2O3 was examined to further enhance the catalytic activity, which gave a drastically improved catalytic activity with T50 = 290 °C. Based on temperature programmed reduction (TPR) and in situ XAFS measurements, the direct oxidation mechanism of soot by the activated lattice oxygen species via the redox of Ag0/Ag2O species was proposed.
中文翻译:

Fe和Ag掺杂的锰氧化物上的柴油机烟尘催化燃烧:杂原子在催化性能中的作用†
采用柠檬酸盐法制备了Fe掺杂的锰氧化物催化剂,并在空气中以紧密接触方式考察了它们在烟灰燃烧中的催化活性。在所测试的催化剂中,的Fe掺杂α-Mn系12摩尔%2 ö 3表现出最高的活性,得到的烟灰燃烧温度(Ť 50 328℃,这与以前对基于稀土报道的)催化剂,而纯α-Mn系2 ö 3给予了Ť 50值339°C。通过X射线衍射(XRD),比表面积测量,氧释放速率测量,X射线光电子能谱(XPS)和X射线吸收精细结构(XAFS)测量进行的综合分析表明,活性的提高归因于i)增加的比表面积和ii)涉及反应性表面吸附的氧种类的改进的氧释放速率。XAFS原位测量表明,Mn和Fe之间的相互作用促进了Mn-O-Fe键的活化并增加了活性氧的比例,从而改善了催化剂的氧化还原性能。此外,同时将Ag和Fe掺杂到Mn 2 O 3中经检测以进一步增强催化活性,这在T 50 = 290°C时得到了显着改善的催化活性。基于程序升温还原(TPR)和原位XAFS测量,提出了活化的晶格氧物种通过Ag 0 / Ag 2 O物种的氧化还原对烟灰的直接氧化机理。
更新日期:2018-02-23
中文翻译:

Fe和Ag掺杂的锰氧化物上的柴油机烟尘催化燃烧:杂原子在催化性能中的作用†
采用柠檬酸盐法制备了Fe掺杂的锰氧化物催化剂,并在空气中以紧密接触方式考察了它们在烟灰燃烧中的催化活性。在所测试的催化剂中,的Fe掺杂α-Mn系12摩尔%2 ö 3表现出最高的活性,得到的烟灰燃烧温度(Ť 50 328℃,这与以前对基于稀土报道的)催化剂,而纯α-Mn系2 ö 3给予了Ť 50值339°C。通过X射线衍射(XRD),比表面积测量,氧释放速率测量,X射线光电子能谱(XPS)和X射线吸收精细结构(XAFS)测量进行的综合分析表明,活性的提高归因于i)增加的比表面积和ii)涉及反应性表面吸附的氧种类的改进的氧释放速率。XAFS原位测量表明,Mn和Fe之间的相互作用促进了Mn-O-Fe键的活化并增加了活性氧的比例,从而改善了催化剂的氧化还原性能。此外,同时将Ag和Fe掺杂到Mn 2 O 3中经检测以进一步增强催化活性,这在T 50 = 290°C时得到了显着改善的催化活性。基于程序升温还原(TPR)和原位XAFS测量,提出了活化的晶格氧物种通过Ag 0 / Ag 2 O物种的氧化还原对烟灰的直接氧化机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号