当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Implications of monomer deformation for tetrel and pnicogen bonds†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1039/c8cp00430g Wiktor Zierkiewicz 1, 2, 3, 4 , Mariusz Michalczyk 1, 2, 3, 4 , Steve Scheiner 5, 6, 7, 8
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-02-23 00:00:00 , DOI: 10.1039/c8cp00430g Wiktor Zierkiewicz 1, 2, 3, 4 , Mariusz Michalczyk 1, 2, 3, 4 , Steve Scheiner 5, 6, 7, 8
Affiliation
|
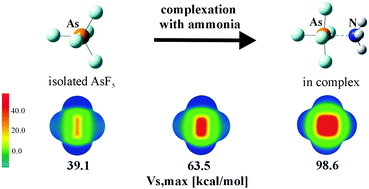
A series of TF4 and ZF5 molecules (T = Si, Ge, Sn and Z = P, As, Sb) were allowed to engage in tetrel and pnicogen bonds, respectively, with NH3, pyrazine, and HCN. The interaction energies are quite large, approaching 50 kcal mol−1 in some cases. The formation of each complex is accompanied by substantial geometrical deformation of the Lewis acid to accommodate the approaching base. The energy associated with this monomer rearrangement is the largest for the smaller central atoms Si and P, where it exceeds 20 kcal mol−1. The total reaction energy of binding, which takes this distortion energy into account, is thus significantly lower than the interaction energy, although remaining quite high, particularly for the larger Sn and Sb central atoms. The tetrel and pnicogen bonds can still form even if the Lewis acid is not permitted to adjust its internal geometry, but they are drastically weakened, dropping by as much as 95%. The monomer rearrangement also aids in the binding by intensifying its σ-hole by a factor of 1.5–2.9.
中文翻译:

蝶形键和pnicogen键的单体变形含义†
允许一系列的TF 4和ZF 5分子(T = Si,Ge,Sn和Z = P,As,Sb)分别与NH 3,吡嗪和HCN接合蝶形键和pnicogen键。相互作用能非常大,在某些情况下接近50 kcal mol -1。每个配合物的形成都伴随有路易斯酸的基本几何变形,以适应即将到来的碱。对于较小的中心原子Si和P,与该单体重排相关的能量最大,在该范围内,能量超过20 kcal mol -1。因此,尽管考虑了这种畸变能,结合的总反应能却大大低于相互作用能,尽管仍然很高,特别是对于较大的Sn和Sb中心原子而言。即使不允许路易斯酸调节其内部几何构型,仍然可以形成三聚氰胺和pnicogen键,但是它们被大大削弱了,最多下降了95%。单体重排还可以通过将其σ孔增大1.5-2.9来辅助结合。
更新日期:2018-02-23
中文翻译:

蝶形键和pnicogen键的单体变形含义†
允许一系列的TF 4和ZF 5分子(T = Si,Ge,Sn和Z = P,As,Sb)分别与NH 3,吡嗪和HCN接合蝶形键和pnicogen键。相互作用能非常大,在某些情况下接近50 kcal mol -1。每个配合物的形成都伴随有路易斯酸的基本几何变形,以适应即将到来的碱。对于较小的中心原子Si和P,与该单体重排相关的能量最大,在该范围内,能量超过20 kcal mol -1。因此,尽管考虑了这种畸变能,结合的总反应能却大大低于相互作用能,尽管仍然很高,特别是对于较大的Sn和Sb中心原子而言。即使不允许路易斯酸调节其内部几何构型,仍然可以形成三聚氰胺和pnicogen键,但是它们被大大削弱了,最多下降了95%。单体重排还可以通过将其σ孔增大1.5-2.9来辅助结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号